Recombinant Human Insulin
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Recombinant DNA Technology
Recombinant human insulin, used for the treatment of diabetes, was the first drug produced using genetic engineering, in 1982. Before this, animals, notably pigs and cattle, were the only non-human sources of insulin.
RECOMBINANT
HUMAN INSULIN
Recombinant
human insulin, used
for the treatment of diabetes, was the first drug produced
using genetic engineering, in 1982.
Before this, animals,
notably pigs and cattle, were the only non-human
sources of insulin. Animal insulin differs
slightly from the human form and,
consequently,
it can potentially elicit an immune response in humans, making
it ineffective. Additionally, the use of recombinant insulin prevents the risks resulting from potential contamination of animal insulin with other
hormones or viruses. To understand how
insulin is produced using recombinant DNA techniques we first need to review its structure. Figure
25.8 illustrates how insulin
is initially synthesized as preproinsulin, a single polypeptide which is processed
during export into proinsulin and finally into active
insulin, once proteolytic cleavage of the connecting sequence
for the two
A and B insulin chains has occurred. These two chains remain
joined through disulphide bonds.
Currently,
different approaches
are employed to produce recombinant insulin,
one of which is shown
in Figure 25.8. First of all, two DNA fragments
coding for the A or the B insulin chains are synthesized chemically.
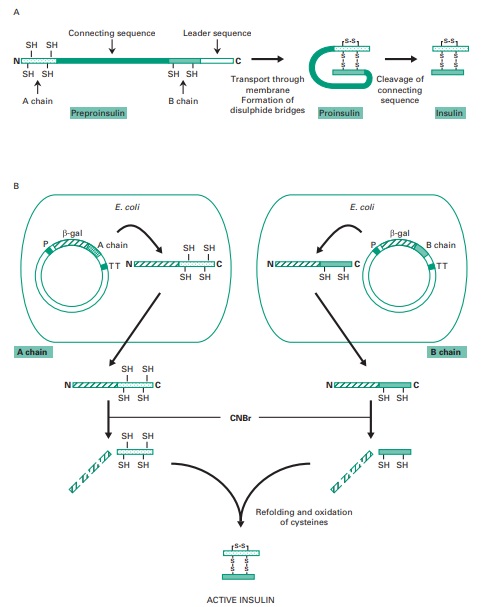
Each of these synthetic
fragments is then individually inserted in a plasmid after the E. coli gene coding
for β galactosidase. This enables this bacterium to produce large fusion proteins
with the insulin
chains tacked on to
the end
of the β-galactosidase enzyme. These fusion
proteins can then be purified from bacterial extracts
and the insulin chains released upon
treatment with cyanogen bromide, which
cleaves peptide bonds
following methionine residues. As methionine was inserted at the boundaries between the β-galactosidase and
the insulin chains, and there
are no methionines present internally within the insulin molecule, treatment with cyanogen bromide results in the cleavage of intact insulin
chains from the fusion proteins. The purified
A and B insulin chains
can be mixed and
reconstituted into an active insulin
molecule. Currently there are also other
methods used to produce recombinant insulin
which are based
on the generation of single
β-galactosidase fusions to the full-length insulin gene containing the coding sequences for both A and B chains. These alternative methods can simplify
the manufacturing of this drug.
Related Topics
