Optimizing Expression of Recombinant Genes
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Recombinant DNA Technology
The primary objective of pharmaceutical companies involved in the production of recombinant drugs is the maximal expression of recombinant genes to generate large quantities of these drugs.
OPTIMIZING EXPRESSION OF RECOMBINANT GENES
The primary objective
of pharmaceutical companies involved in the production of recombinant drugs
is the maximal expression of recombinant genes to generate large quantities of these drugs.
Unfortunately, the cloning of a gene into a vector does not ensure
that it will be properly expressed. To improve
expression of a cloned
gene the different stages that
lead to the synthesis of the
protein therefore have
to be optimized. This is achieved
by the use of so-called expression vectors
(Figure 25.6). Some expression vectors have
been designed to produce large quantities of protein in specific cell hosts. For example, the bacmids are shuttle
expression vectors derived from a baculovirus ds DNA circular genome
and are used to transfect insect
cells in order
to produce large quantities of a recombinant protein
in fermenters.
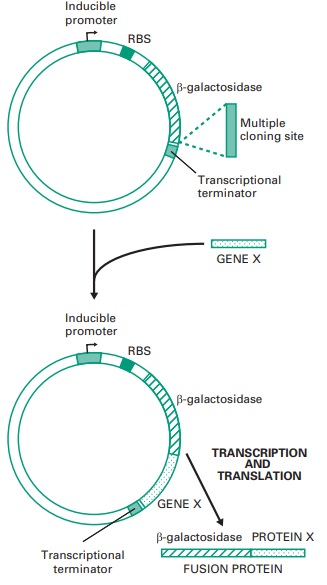

A)
Optimizing Transcription
To optimize transcription it must be ensured that the recombinant gene is placed after a promoter
(Figure 25.6) that will be recognized by the RNA polymerase of the host cell where the gene is going to be expressed. Two types of promoters
can be used:
(1) constitutive promoters, which are expressed all the time and (2) inducible promoters, where expression is turned off during culture growth and turned
on, for example, upon the addition
of an inducing molecule to the culture, usually shortly before harvesting, when high numbers
of bacteria are present in the culture. Inducible promoters are very useful when expressing foreign genes coding
for proteins toxic to the bacterial hosts as their premature expression could lead to growth impairments and consequently low yields of recombinant protein.
Furthermore, to ensure that transcription finishes
after the 3′ end of the recombinant gene, a transcriptional terminator (Figure 25.6) must
be placed just
downstream of this gene.
B)
Optimizing Translation
A key feature that
determines whether a gene is going to be efficiently translated by a certain
host is the nucleotide sequence of the ribosome
binding site (RBS), located upstream of the gene (Figure
25.6), which needs to be efficiently recognized by the ribosomes of this host. In
addition, the distance between the RBS and the translation start codon needs to be optimal
to enable the right interactions between the mRNA
and the ribosomes and start the protein
synthesis. There are commercially
available vectors
carrying sequences for RBSs and
translation start
codons which are optimally recognized by the ribosomes of the host cells, ensuring
that any recombinant genes cloned after
the start codon
will be maximally translated.
Small proteins are frequently susceptible to proteolytic degradation
when expressed in a foreign
host. This degradation can be avoided
by expressing them fused to a
larger protein.
This is normally
achieved by cloning the small gene downstream of a
gene coding for a protein such as β-galactosidase.
To obtain
the fusion protein (Figure 25.6)
it is essential to ensure
that the reading frame is conserved and that no translation stop codons are present between the β-galactosidase
and the target gene, enabling the
ribosomes to read through. Interestingly, affinity columns that will bind the fused polypeptides are available, which facilitates the purification of the recombinant protein by affinity chromatography.
C) Post-Translational Modifications
Although high levels of
protein production may be achieved by optimizing transcription and translation of a gene, the obtained protein
may still need to undergo post-translational modifications before it is
active. Some of these
modifications include correct
disulphide bond formation, proteolytic cleavage of a precursor, glycosylation and additions to amino acids
such as phosphorylation, acetylation, sulphation, acylation, etc. Unfortunately, the practical E. coli
host, in which
most recombinant proteins are produced,
does not possess
the same type of
cellular machinery
required for these modifications. Recently, Campylobacter jejuni has been found
to possess an eukaryotic-like system
for protein glycosylation and efforts are being made to genetically engineer
E. coli strains to perform
the adequate glycosylation of recombinant proteins as mammalian cells.
Hence, it is essential
to select
a suitable host for the expression of the target gene that can carry
out the required post-translational modifications that will enable the synthesis of large
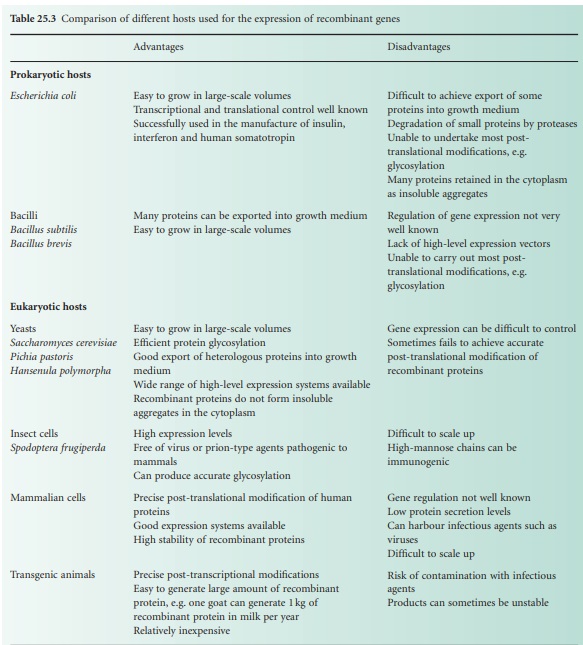
amounts of a biologically authentic product. Table 25.3 shows a comparison of a selection
of hosts currently
used for the expression of recombinant proteins.
Related Topics
