Recombinant Somatotropin
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Recombinant DNA Technology
Somatotropin, also known as human growth hormone (hGH), consists of 191 amino acids; it is produced in the pituitary gland and regulates growth and development. Regular injections of hGH are given to children with dwarfism caused by the lack of this hormone so that they can reach near normal heights.
RECOMBINANT SOMATOTROPIN
Somatotropin, also known as human growth
hormone (hGH), consists of 191 amino
acids; it is produced in the
pituitary gland and
regulates growth and development.
Regular injections of hGH are given to children with dwarfism caused by the lack
of this hormone
so that they can reach near normal
heights. In this
case, unlike with insulin, animal-derived hormones
are ineffective and only the human protein
works. Because of continuous
shortages of pituitaries from human
cadavers, the production of recombinant hGH has been imperative.
Furthermore, the contamination of children with fatal
viruses from the cadavers
has been an additional reason for moving away
from this source
of hormone. As the
gene for the hGH is 573 nucleotides long,
it was in practice too
large to be synthetically produced to generate the recombinant hormone as achieved with insulin and somatostatin. Hence there have been two
practical ways of generating recombinant hGH, one of which resulted
in the generation of this
hormone with an added methionine at the N-terminus. Figure 25.10 shows these two strategies.
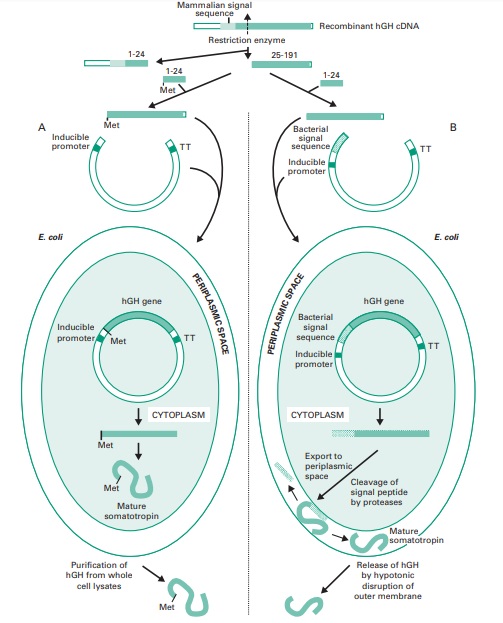
Initially the coding region
for hGH was isolated from a
cDNA library. The DNA fragment coding
for the mammalian signal peptide
can then be excised by a restriction enzyme that also removes the first 24 codons of the
mature protein. A
chemically synthesized DNA fragment containing
a methionine codon,
to enable translation in E.
coli, followed by these first 24 codons was therefore
ligated to the DNA
fragment coding for
the remaining amino acids
25–191 of the hGH (Figure
25.10A). The resulting DNA was cloned
into an expression vector and transformed into E. coli
where the recombinant hGH accumulated in the cytoplasm. The recombinant hormone can be isolated from
bacterial cell extracts and, in contrast to the non-recombinant protein, carries
a methionine residue at the N-terminus.
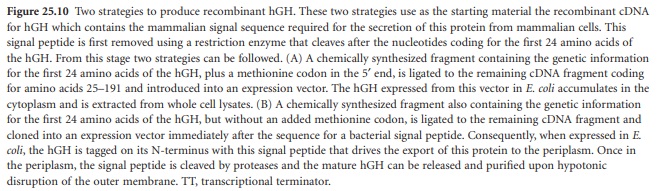
Figure 25.10B
shows an alternative method consisting of the replacement of the mammalian signal peptide for a secretion signal peptide functional
in bacteria. This enables the purification of the recombinant hGH from the periplasm of the bacterial cell, reducing the difficulties associated with the purification of
recombinant proteins from the cytoplasm. To achieve this, once the mammalian signal peptide
has been removed
as above, a synthetic DNA molecule containing the missing 24 codons of the hGH, without
an added methionine codon, is ligated to the DNA fragment coding
for the 25–191 remaining residues. The
resulting DNA molecule is then inserted in an expression vector that codes for a bacterial
signal peptide
in fusion with
the hGH gene.
Once transformed into E. coli, the recombinant hGH is produced and the signal sequence
will target the protein for secretion into
the periplasmic space
where it will
accumulate. The periplasmic proteases release the signal peptide, leaving hGH without
additional amino acids.
The protein can then be easily purified
from the periplasmic space after release by hypotonic disruption of the outer membrane.
Related Topics
