Endogenous Opioid Peptides
| Home | | Pharmacology |Chapter: Essential pharmacology : Opioid Analgesics And Antagonists
In the mid 1970s, with herculean efforts, a number of peptides having morphinelike actions were isolated from mammalian brain, pituitary, spinal cord and g.i.t.
ENDOGENOUS OPIOID PEPTIDES
In the mid 1970s, with
herculean efforts, a number of peptides having morphinelike actions were
isolated from mammalian brain, pituitary, spinal cord and g.i.t. These are
active in very small amounts, their actions are blocked by naloxone, and they
bind with high affinity to the opioid receptors. There are 3 distinct families
of opioid peptides. Each is derived from a specific large precursor
polypeptide.
Endorphins
β-endorphin (βEND) having amino acids is the most important of the
endorphins. It is derived from Proopiomelanocortin (POMC) which also gives rise
to γ-MSH, ACTH and two
lipotropins.
βEND
is primarily μ agonist, but also has
δ action.
Enkephalins
Methionine-enkephalin
(met-ENK) and leucine-enkephalin (leu-ENK) are the most important. Both are
pentapeptides. The large precursor peptide proenkephalin
has 4 met-ENK and 1 leu-ENK residues. The two ENKs have a slightly different
spectrum of activity; while met-ENK has equal affinity for μ and δ sites, leu-ENK
prefers δ receptors.
Dynorphins
Dynorphin A and B (DYNA, DYNB) are 8–17 amino acid peptides derived
from prodynorphin which contains 3
leu-ENK residues also. DYNs are more potent on κ receptors, but also
activate μ and δ receptors.
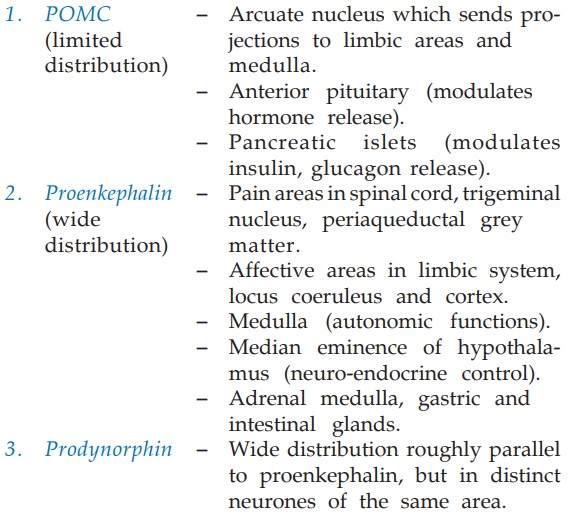
Distribution of the 3 families of peptides is summarized
below:
The opioid peptides constitute an endogenous opioid system which
normally modulates pain perception, mood, hedonic (pleasure related) and motor behaviour,
emesis, pituitary hormone release and g.i.t. motility, etc.
β-END injected directly
into the brain is 20– 40 times more potent analgesic than morphine.
Its primary localization in hypothalamus and pituitary and its long t½ ascribes
it a neurohormone function which
modulates the release of other
hormones. It decreases LH, FSH release and increases GH and prolactin release.
Naloxone has opposite effects on the levels of these hormones—suggesting that
the system is constitutively active.
The wide distribution of ENKs and DYNs and their short t½
suggests function as neuromodulator or neurotransmitter. They appear to regulate pain responsiveness at spinal
and supraspinal levels. Naloxone blocks placebo, acupuncture and stress-induced
analgesias, suggesting the involvement of opioid peptides in these responses.
Opioid peptides also appear to participate in regulation of affective behaviour
and autonomic function.
Recently a novel opioid peptide Nociceptin/orphanin FQ (N/OFQ)
has been isolated from mammalian brain. It is localized in cortex, hippocampus,
spinal cord and certain sensory sites; is believed to play a role in stress
response, reward and reinforcing actions, learning and memory. The N/OFQ
receptor, also labelled ‘Opioid-receptor-like1’ (ORL1) receptor, is thus the 4th
opioid receptor to be identified. At certain sites, N/OFQ can act as an
‘antiopioid’ through the ORL1 receptor. In the pain control mechanisms, N/OFQ
appears to play both opioidlike as well as antagonistic roles, depending on the
site and the basal state of pain.
Morphine and other opioids act as exogenous agonists on some of
the receptors for these peptides. This has given an explanation for the
existence of specific receptors in the body for exogenous substances like morphine.
Morphine itself has now been detected in mammalian brain.
Related Topics
