Exercises - The Boron Group - Group 13
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : The Boron Group - Group 13
Inorganic Chemistry : The Boron Group - Group 13: Exercises
Exercises
1. Draw the Lewis
structure or chemical formula of the following aluminium-based drugs
(a)
Aluminium acetate
(b)
Aluminium chloride
(c)
Aluminium oxide
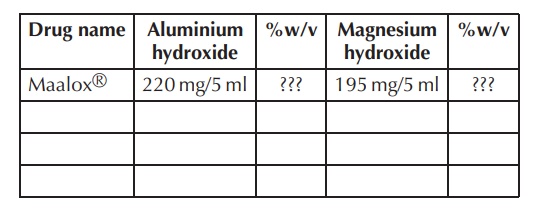
2. Research
different antacids mixtures, state their content and calculate the
weight/volume per-centage (%w/v) for each active pharmaceutical ingredient
(API).
3. A typical
antacid capsule contains 475 mg aluminium hydroxide as the active ingredient.
How many milligrams of stomach acid (HCl) can be neutralised by
one tablet?
4. An
aluminium hydroxide suspension (30 ml) containing 500 mg/5 ml aluminium
hydroxide is prescribed to the patient. The prescription states the patient has
to take 30 ml four times a day.
(a)
What is the chemical formula of aluminium
hydroxide?
(b)
How many grams of Al3+ is given to
the patient per single dose?
(c)
What is the weight/volume percentage (%w/v) of
aluminium hydroxide in the suspension?
5. Write the
chemical equations explaining the amphoteric behaviour of gallium hydroxide.
Related Topics
