Facilities and equipment
| Home | | Hospital pharmacy |Chapter: Hospital pharmacy : Technical services
All aseptic manipulation must take place in EU grade A work zones. These can be provided by horizontal or vertical laminar air flow cabinets or positive-pressure isolators.
Facilities and equipment
All aseptic
manipulation must take place in EU grade A work zones. These can be provided by
horizontal or vertical laminar air flow cabinets or positive-pressure
isolators. For hazardous drugs, such as cytotoxics and radiopharma-ceuticals,
the use of a negative-pressure isolator is recommended to provide protection
not only to the product, but also the operator(s). The grade A workstation must
be located in a controlled background environment, usually of EU grade B,
although some isolators may be located in an EU grade C or D background. Figure
6.4 shows horizontal laminar flow workstations used for IV additive work.

Automated filling
equipment may be placed in the critical work zone providing it is fully
validated. Figure 6.5 shows an automated system.

The Rules and
Guidance for Good Pharmaceutical Manufacture and Distribution and Quality
Assurance of Aseptic Preparation Services should be consulted for exact
standards and requirements of facilities and equipment. Specialist guidance on
isolator technology is also available.
In addition to the
critical aseptic handling areas, areas must be desig-nated for setting up
ingredients, producing batch documents and labels, and checking and packing the
finished product. Handling radiopharmaceuticals requires additional equipment
to protect the operator from ionising radia-tion and to monitor exposure
levels. Also, tandem isolator systems are necessary to include the technetium
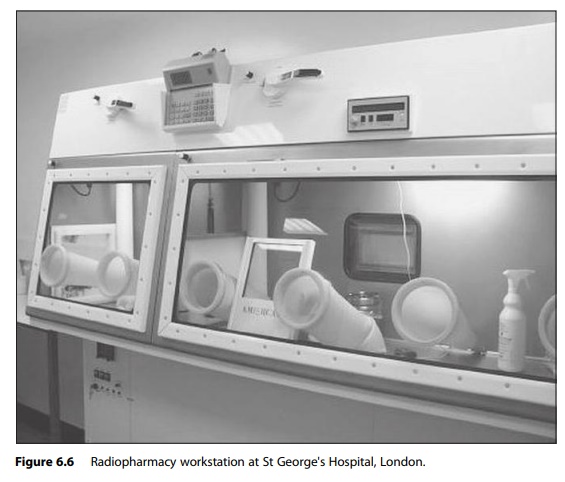
(Tc99) generator in the controlled work area (Figure 6.6). Operators are required
to wear body badges and finger badges in order to quantify the amount of
exposure they have received. They must also follow systems of work which
control exposure by either mini-mising the time spent directly exposed to the
source of radiation or by maximising the distance from it. Such practices
include working behind lead glass shields and the use of syringe and vial
shields and lead housing for generators. These shields must also be
accommodated within the isolator or class 2 cabinet workstation. Tongs are used
to increase the distance between the operator and the doses. Dose monitors are
also used to check for spil-lages and contamination and are subsequently used
to ensure such incidents are cleared up appropriately. There are specific
elements of operator training which must be covered besides the routine
pharmacy training. There are also local rules which must be read which cover
the safe systems of working with radiation. The introduction of the robotic arm
for manipulation of radio-pharmaceuticals should further increase operator
safety.
Process
Wherever possible,
all aseptic processes should be based on closed systems so that the product or
the product fluid path has only minimal exposure to the environment. Product
segregation is essential to prevent gross contamin-ation and separate clean
rooms and workstations should be used for cytotoxic drugs and
radiopharmaceuticals. Operator technique is critical and all opera-tors, processes
and equipment must be fully validated.
The manipulation of
cytotoxic drugs requires additional protective cloth-ing and emergency
procedures for spillage management. These are detailed in non-official UK
guidelines. To avoid aerosol formation, venting nee-dles and filters or
purpose-designed fluid transfer devices must be used when adding and
withdrawing liquids to and from vials. In the case of aseptic products,
environmental monitoring and the use of routine media-fill simula-tions are
more meaningful than sterility tests, which are designed to be used with
terminally sterilised medicines. Support from an experienced QA depart-ment is
essential not only for the validation of all aseptic processes but also for
formulation and shelf-life issues with aseptic preparations. The addi-tional
risks associated with unintentional intrathecal administration of cer-tain
cytotoxic agents have led to additional guidance on the presentation, process
and release of these products. With a few exceptions, all vinca alkal-oid doses
must now be presented as large-volume infusions (50 ml) to prevent the lethal
intrathecal administration of these drugs.
The processes
involved in the preparation of radiopharmaceuticals require consideration of
additional issues, including the prescribing and scheduling doses, which are
often complex. Most doctors who request scans are not authorised to prescribe
radioactive pharmaceuticals. Requests must therefore be authorised by the local
Administration of Radioactive Substances Advisory Committee licence holder
before they can be scheduled into the nuclear medicine clinic. To maximise the
scanning capacity of a nuclear medicine department, doses need to be ready at
the beginning of the working day. An on-site radiopharmacy can help facilitate
this and enable the service to be more flexible when responding to urgent
requests. In contrast to routine CIVAS work, radiopharmacy staff start dose
preparation first thing in the morning and early starts of 7 a.m. are not
unusual. Reconstitution of kits requires the addition of a radionuclide and
saline to a ligand contained in a sterile vial. The resulting solution may need
to be incubated for a set period to ensure the radionuclide has attached to the
ligand. Simple QC analysis can be performed to confirm the radiochem-ical
purity of the radiopharmaceutical. Poor-quality radiopharmaceuticals may expose
patients to radiation unnecessarily and their treatment may be delayed while
the investigation is repeated.
Related Topics
