Female Contraception - Hormonal Contraceptives
| Home | | Pharmacology |Chapter: Essential pharmacology : Estrogens, Progestins and Contraceptives
Over 100 million women worldwide are currently using hormonal contraceptives. With these drugs, fertility can be suppressed at will, for as long as desired, with almost 100% confidence and complete return of fertility on discontinuation.
FEMALE CONTRACEPTION
Over 100 million women
worldwide are currently using hormonal contraceptives. With these drugs,
fertility can be suppressed at will, for as long as desired, with almost 100%
confidence and complete return of fertility on discontinuation. The efficacy,
convenience, low cost and overall safety of oral contraceptives (OCs) has
allowed women to decide if and when they will become pregnant and to plan their
activities. A variety of oral and parenteral preparations are now available
offering individual choices.
Types Of Methods
Oral
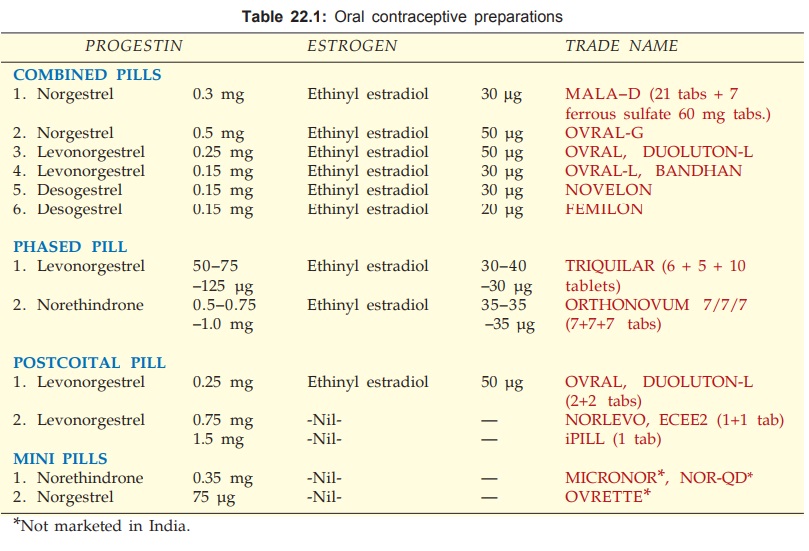
1. Combined Pill
It
contains an estrogen and a progestin. With
accumulated experience, it has been possible to reduce the amount of estrogen
and progestin in the ‘second generation’ OC pills without compromising efficacy,
but reducing side effects and complications. ‘Third generation’pills containing
newer progestins like desogestrel with improved profile of action have been
introduced in the 1990s. Ethinylestradiol 30 μg daily is considered
threshold but can be reduced to 20 μg/day if a progestin with potent anti-ovulatory action is included.
The progestin is a 19 nortestosterone because these have potent anti-ovulatory
action. Used alone the ovulation inhibitory dose (per day) of the currently
used progestins is estimated to be—levonorgestrel 60 μg, desogestrel 60 μg, norgestimate 200 μg, gestodene 40 μg, but the amount in
the pill is 2–3 times higher to attain 100% certainty. While both estrogens and
progestins synergise to inhibit ovulation, the progestin ensures prompt
bleeding at the end of a cycle and blocks the risk of developing endometrial
carcinoma due to the estrogen. One tablet is taken daily for 21 days, starting
on the 5th day of menstruation. The next course is started after a gap of 7
days in which bleeding occurs. Thus, a cycle of 28 days is maintained. Calendar
packs of pills are available. This is the most popular and most efficacious
method.
2. Phased Regimens
These have been introduced to permit reduction
in total steroid dose without compromising efficacy. These are biphasic or
triphasic. The estrogen dose is kept constant (or varied slightly between 30–40
μg), while the amount
of progestin is low in the first phase and progressively higher in the second and
third phases.
Phasic
pills are particularly recommended for women over 35 years of age or when other
risk factors are present.
3. Minipill (Progestin Only Pill)
It has been devised to eliminate the estrogen, because
many of the long-term risks have been ascribed to this component. A lowdose
progestin only pill is taken daily continuously without any gap. The menstrual
cycle tends to become irregular and ovulation occurs in 20–30% women, but other
mechanisms contribute
to the contraceptive action. The efficacy is lower (96–98%) compared to
98–99.9% with combined pill—look for pregnancy if amenorrhoea of more than 2
months occurs. This method is less popular.
4. Postcoital (Emergency)
Contraception Currently 3 regimens are available:
a.
Levonorgestrel 0.5 mg + ethinylestradiol 0.1
mg (2 OVRAL tablets) taken as early as possible but within 72 hours of
unprotected intercourse and repeated after 12 hours. Till recently, this
regimen called the ‘Yuzpe method’ has been the most popular. It is estimated to
prevent 3 out of 4 possible pregnancies, but nearly 50% women experience nausea
and 20% vomit.
b.
Levonorgestrel alone 0.75 mg taken twice with
12 hour gap within 72 hours of intercourse.
Trials conducted
globally by the WHO taskforce on postovulatory methods of fertility control
have found this regimen to be 2–3 times more effective and better tolerated.
Incidence of vomiting is only 6% and other side effects are also less. However,
the next period may be some what delayed. The WHO essential drug list (2001) recommended
replacement of Yuzpe method by this regimen.
c. Mifepristone 600 mg single dose taken
within hours of intercourse has been used in China, Europe and few other
countries with high success and fewer side effects than Yuzpe method.
Emergency postcoital
contraception should be reserved for unexpected or accidental exposure (rape,
condom rupture) only, because all regimens have higher failure rate and side
effects than regular lowdose combined pill.
Injectable
These have been
developed to obviate need for daily ingestion of pills. They are given i.m. as
oily solution; are highly effective; over 50 million women have used them so
far. Their major limitations are:
a. Animal data has
indicated carcinogenic potential but there is no proof yet from human studies despite
30 years of experience. No increase in overall risk of cervical, ovarian or
hepatic cancer has been noted by a WHO sponsored study. Breast cancer risk may
be slightly increased in younger women (< 35 yr). The logistics of
administration and supervision for mass use are considered inadequate in
developing countries. Use effectiveness in field conditions is low. In India
approval has been granted for use only under close supervision, but not on mass
scale under the National Programme.
b. Menstrual irregularities, excessive
bleeding or amenorrhoea are very common; incidence of amenorrhoea increases
with increasing duration of use. Return of fertility may take 6–30 months after
discontinuation; permanent sterility may occur in some women. Weight gain and
headache occur in >5% subjects. Bone mineral density may decrease in long-term
users due to low estrogen levels caused by Gn suppression. This may also
produce menopauselike symptoms (hot flushes, vaginal dryness, reduced libido).
Two types of
preparations have been tested:
i) Long acting progestin alone—injected once in 2–3 months depending on the steroid and its amount. Two compounds have been marketed:
a.
Depot medroxyprogesterone acetate (DMPA) 150 mg at 3month
intervals. After i.m. injection peak blood levels are reached in weeks and
decline with a t½ of ~ 50 days.
DEPOTPROVERA
150 mg in 1 ml vial for deep i.m. injection during first 5 days of menstrual
cycle. Repeat every 3 months.
b. Norethindrone (Norethisterone) enanthate (NEE) 200 mg
at 2month intervals.
NORISTERAT
200 mg in 1 ml vial for deep i.m. injection during first 5 days of menstrual
cycle. Repeat every 2 months.
The
most important undesirable property is complete disruption of menstrual
bleeding pattern and total amenorrhoea (more common with DMPA). It is not
suitable for adolescent girls and lactating mothers. Use of DMPA is generally
restricted to women who are unlikely to use other contraceptives effectively.
NEE is shorter acting and failure rates have been higher than with DMPA.
ii)
Long
acting progestin + long acting estrogen — once a month. These have been tested to
a more limited extent, but a combination of MPA + estradiol cypionate has been
approved by USFDA for i.m. injection every month. Main advantage is that they
allow a reasonable menstrual bleeding pattern in most cases. Their obvious
disadvantage is that they contain a long acting estrogen which has potential to
harm.
All
fixed dose combination injectable preparations of synthetic estrogens and
progestins are not allowed in India.
Implants
These are drug
delivery systems implanted under the skin, from
which the steroid is released slowly over a period of 1–5 years. They consist
of either—
i)
Biodegradable polymeric matrices—do not need to be
removed on expiry.
ii)
Nonbiodegradable rubber membranes—have to be removed on
expiry.
NORPLANT:
A
set of 6 capsules each containing 36 mg levonorgestrel (total
216 mg) for subcutaneous implantation is available in some countries, but has
been discontinued in the USA. Works for up to 5 years.
A progesterone
impregnated intrauterine insert (PROGESTASERT) has been introduced in
some countries. It contains lesser quantity of progestin which primarily acts
locally on endometrium. The device is to be replaced yearly and efficacy is
rated lower.
Mechanism
Of Action
Hormonal
contraceptives interfere with fertility in many ways; the relative importance
depends on the type of method. This is summarized in Table 22.2.
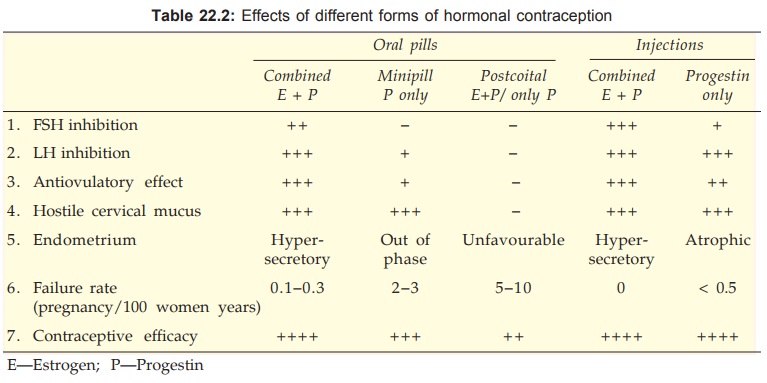
1. Inhibition of Gn release from pituitary by reinforcement of
normal feedback inhibition. The progestin reduces frequency of LH secretory
pulses (an optimum pulse frequency is required for tiggering ovulation) while
the estrogen primarily reduces FSH secretion. Both synergise to inhibit
midcycle LH surge. When the combined pill is taken both FSH and LH are reduced
and the midcycle surge is abolished. As a result, follicles fail to develop and
fail to rupture— ovulation does not occur.
The minipill and progestin
only injectable regimen also attenuate LH surge but less consistently—ovulation
occurs in ~ 1/3 cycles. However, pregnancy is still prevented by direct actions
on the genital tract.
2. Thick cervical mucus
secretion hostile to sperm penetration is evoked by progestin action. As such, this mechanism can operate with all methods except postcoital
pill.
3. Even if ovulation and fertilization occur, the blastocyst may
fail to implant because endometrium is
either hyperproliferative or hypersecre
tory
or atrophic and in any case out of phase
with fertilization—not suitable for nidation. This action appears to be most
important in case of minipills and postcoital pill.
4. Uterine and tubal
contractions may be modified to disfavour fertilization. This action
is uncertain but probably contributes to the efficacy of minipills and
postcoital pill.
5. The postcoital pill may dislodge a just implanted blastocyst or may interfere with
fertilization/ implantation.
Practical Considerations
Ø Discontinuation of all
OCs results in full return of fertility within 1–2 months. There may even be a
rebound increase in fertility—chances of multiple pregnancy are more if conception
occurs within 2–3 cycles. With injectable preparations, return of fertility is
delayed. The cycles take several months to normalize or may not do so at all.
They are to be used only if the risk of permanent infertility is acceptable.
Ø If a woman on combined
pills misses to take a tablet, she should be advised to take two tablets the
next day and continue as usual. If more than
Ø tablets are missed,
then the course should be interrupted, an alternative method of contraception
used and next course started on the 5th day of bleeding.
Ø If pregnancy occurs
during use of hormonal contraceptives—it should be terminated by suctionaspiration,
because the risk of malformations, genital carcinoma in female offspring and
undescended testes in male offspring is increased.
Ø While for most women a
pill containing 30 μg ethinylestradiol is sufficient, the obese may require one
containing 50 μg, while those above
Ø yr age may do with 20 μg.
Ø If breakthrough
bleeding occurs—switch over to a pill containing higher estrogen dose.
Ø In women with
contraindications for estrogen (see
below), a progestin only contraceptive may be used.
Ø For women who develop
weight gain, acne or raised LDL cholesterol due to the androgenic action of the
older 19nortestosterone progestin— a newer progestin (e.g. desogestrel) lacking
androgenic action may be preferable.
Adverse Effects
Since
contraceptives are used in otherwise healthy and young women, adverse effects,
especially long-term consequences assume great significance. The adverse
effects are dose dependent; most of the past data with highdose preparations
cannot be directly extrapolated to the presentday lowdose preparations which
carry relatively minor risk. The following applies primarily to combined oral
pill which has been most extensively used.
A. Nonserious Side Effects
These
are frequent, specially in the first 1–3 cycles and then disappear gradually.
Ø Nausea and vomiting:
similar to morning sickness of pregnancy.
Ø Headache is generally
mild; migraine may be precipitated or worsened.
Ø Breakthrough bleeding
or spotting: specially with progestin only preparations. Amenorrhoea may occur
in few, or the cycles may get disrupted: especially with injectables and
minipill.
Ø Breast discomfort.
B. Side Effects That Appear Later
Ø Weight gain, acne and
increased body hair may be noted due to androgenic action of older 19nortestosterone
progestins. The newer ones like desogestrel lack this effect.
Ø Chloasma: pigmentation
of cheeks, nose and forehead, similar to that occurring in pregnancy.
Ø Pruritus vulvae is
infrequent.
Ø Carbohydrate intolerance
and precipitation of diabetes in few subjects taking high dose preparations;
but this is unlikely with the present pills. Many large studies have found no
link between OC use and development of diabetes.
Ø Mood swings, abdominal
distention are occasional; especially reported with progesterone only
contraceptives.
C. Serious Complications
1.
Leg vein and pulmonary thrombosis:
The older preparations
increased the incidence of venous thromboembolism, but this is found to be only
marginal with the newer reduced steroid content pills. However, even these pose
significant risk in women >35 years of age, diabetics, hypertensives and
those who smoke. The risk normalizes shortly after stopping the OC.
2.
Coronary and cerebral thrombosis
resulting in myocardial infarction
or stroke: A 2 to 6fold increase in
risk was estimated earlier, but recent studies have found no increased
incidence with the low dose pills in the absence of other risk factors.
The
estrogen component of OC has been mainly held responsible for venous thromboembolism,
while both estrogen and progestin have been blamed for the arterial phenomena.
The mechanisms involved may be:
o Increase in blood
clotting factors (coagulability is enhanced).
o Decreased antithrombin
III.
o Decreased plasminogen
activator in endothelium.
o Increased platelet
aggregation.
3. Rise in BP: occurred in 5–10%
women taking the earlier pills. This
again is less frequent and smaller in magnitude with the lowdose pills of
today. If the BP rises, best is to stop OCs—BP normalizes in the next 3–6
months. Both the estrogen and progestin components are responsible for this
effect, probably by increasing plasma angiotensinogen level and renin activity
which induces salt and water retention.
4. Estrogen tends to raise plasma HDL/LDL ratio (beneficial),
but the progestin nullifies this benefit: lipid profile is not significantly
altered by low dose OCs, except that triglyceride level may rise marginally
which poses no excess risk.
5. Genital carcinoma: an increased incidence
of vaginal, cervical, and breast
cancers was feared on the basis of animal data, but extensive epidemiological
data over the past 30 years has repeatedly shown that oral as well as injected
contraceptives do not increase the occurrence of these cancers in the general
population. How ever, risk is increased in predisposed individuals. Growth of
already existing hormone dependent tumour may be hastened.
Epidemiological data
has recorded minor increase in breast cancer incidence among current OC users,
but not among past users. Since breast cancer is rare in young women, this
finding is considered inconsequential.
A protective effect
against endometrial carcinoma has been shown for the progestin component.
Prolonged suppression of gonadotropic stimulation of ovary may account for the
lower incidence of ovarian malignancy noted in contraceptive users.
6. Benign hepatomas:
which may rupture or turn malignant;
incidence of this rare tumour appears to be slightly higher in OC users.
7. Gallstones: Estrogens increase biliary
cholesterol excretion; incidence of gallstones is slightly higher in women who
are taking OCs, or after long-term use.
Contraindications
The combined oral
contraceptive pill is absolutely contraindicated in:
Ø Thromboembolic,
coronary and cerebrovascular disease or a history of it.
Ø Moderatetosevere
hypertension; hyperlipidaemia.
Ø Active liver disease,
hepatoma or h/o jaundice during past pregnancy.
Ø Suspected/overt malignancy of genitals/ breast.
Ø Prophyria.
Ø Impending major
surgery—to avoid postoperative thromboembolism.
Relative Contraindications
(requiring avoidance/ cautious use under supervision)
Ø Diabetes: control may
be vitiated.
Ø Obesity
Ø Smoking
Ø Undiagnosed vaginal
bleeding
Ø Uterine leiomyoma: may
enlarge with estrogenic preparations; progestin only pills can be used.
Ø Mentally ill
Ø Age above 35 years
Ø Mild hypertension
Ø Migraine
Ø Gallbladder disease
Interactions
Contraceptive
failure may occur if the following drugs
are used concurrently:
a) Enzyme inducers: phenytoin, phenobarbitone, primidone, carbamazepine, rifampin. Metabolism of estrogenic as
well as progestational component is increased.
b) Suppression of intestinal microflora: tetracyclines, ampicillin, etc. No deconjugation of
estrogens excreted in bile → their enterohepatic circulation is interrupted
→ blood levels fall.
With
both types of interacting drugs, it is wise to switch over to a preparation
containing 50–80 μg of ethinylestradiol or to use alternative method of contraception.
Other Health Benefits
Apart from benefits
due to prevention of unwanted
pregnancy and the risks during delivery, use of oral contraceptives affords
certain other beneficial effects as a bonus:
Ø Lower probability of
developing endometrial and ovarian carcinoma; probably colorectal cancer as
well.
Ø Reduced menstrual
blood loss and associated anaemia; cycles if irregular become regular;
premenstrual tension and dysmenorrhoea are ameliorated.
Ø Endometriosis and
pelvic inflammatory disease are improved.
Ø Reduced incidence of
fibrocystic breast disease and ovarian cysts.
Centchroman
It
is a nonsteroidal estrogen antagonist or SERM
developed at CDRI, India and introduced in the National Family Welfare
Programme as an oral contraceptive. It probably acts as an anti-implantation
agent by inducing embryo uterine asynchrony, accelerated tubal transport and
suppression of decidualization. It prevents conception as long as taken, with
return of fertility on withdrawal. Failure rate of 1–3% has been recorded.
Pituitary, ovarian and other endocrine functions do not appear to be affected.
The menstrual cycle is
not disturbed in most women, but in 6–10% it may be lengthened irregularly.
The usual side effects
of hormonal contraceptives have not been noted. No derangement of laboratory
tests including blood sugar and lipid profile have been detected. No
teratogenic, mutagenic or carcinogenic effect has been observed so far.
However, if pregnancy occurs centchroman should be discontinued immediately.
Centchroman has a long
plasma t½ (about 1 week). The recommended dose is 30 mg twice weekly for 12
weeks followed by once a week as long as fertility is to be suppressed. More
experience has to be gained to decide its role as a contraceptive.
CENTRON, SAHELI 30 mg
tab.
Related Topics
