Ferrocene
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Organometallic Chemistry
1. Ferrocene and its derivatives as biosensors, 2. Ferrocene derivatives as potential antimalarial agent , 3. Ferrocifen – a new promising agent against breast cancer?
Ferrocene
Ferrocene (or bis(η5-cyclopentadienyl)iron, (C5H5)2Fe)
is an orange powder and is probably one of the best studied metallocenes. As
previously mentioned, its structure follows the 18-electron rule and it is a
very stable complex. Its Cp− ligands can be easily derivatised to
introduce functional groups. Functionalised ferrocene derivatives are currently
used as biosensors in blood glucose measuring equipment and they are also under
intense research as potential anticancer agents.
Ferrocene was discovered by Paulson and Kealy in 1951.
Cyclopentadienyl magnesium bromide and ferric chloride were reacted in a
so-called Grignard reaction (reaction involving R-MgBr) in order to create a
fulva-lene. Instead, they created ferrocene. At that time, it was difficult to
identify the correct structure of ferrocene, but Wilkinson, Rosenblum, Whitting
and Woodward managed to do this soon after its discovery .
2C5H5MgBr + FeCl2 → Fe(C5H5)2
+ MgBr2 + MgCl2
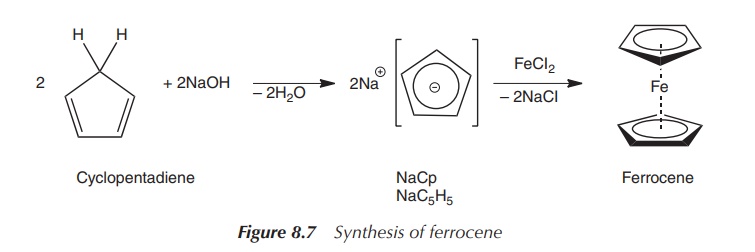
Nowadays, ferrocene is synthesised via a so-called
transmetallation reaction. Typically, commercially available sodium
cyclopentadienide is deprotonated with KOH or NaOH, and the obtained anion is
reacted with anhydrous ferrous chloride (FeCl2). Instead of
purchased sodium cyclopentadienide, freshly cracked cyclopentadiene is often
used (Figure 8.7).
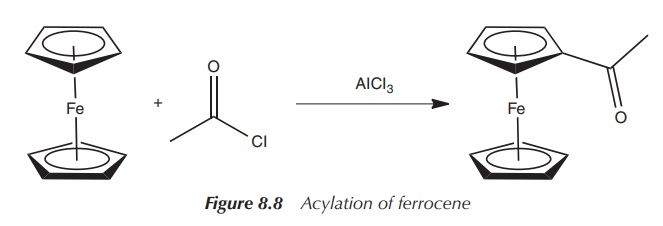
Ferrocene is a very stable complex and can be easily
functionalised by derivatising its Cp− ligands. The Cp−
ligands are aromatic, as previously mentioned, and therefore show a chemical
behaviour similar to benzene. This means that reactions known for benzene
chemistry can be used with ferrocene, such as the Friedel–Crafts acylation
reaction. Ferrocene can be acylated by reacting it with the corresponding
aluminium halide (AlX3). Indeed, this chemical behaviour of
ferrocene helped in identifying its real structure (Figure 8.8) .
Ferrocene and its derivatives are under intense screening for medicinal purposes. Research has shown that especially ferrocene derivatives exhibit very promising effects for a variety of clinical applications, such as antimalarial and anticancer agents as detailed below. Interestingly enough, ferrocene itself is not a particularly toxic compound, as it can be administered orally, injected or inhaled with no serious health concerns. It is believed to be degraded in the liver by cytochrome P450, similar to benzene. Its degradation process involves the enzymatic hydroxylation of the cyclopentadienyl ligand. Animal studies on beagles have shown that treat-ment with up to 1 g/kg ferrocene did not result in acute toxicity or death, although it did lead to a severe iron overload, which was reversible .
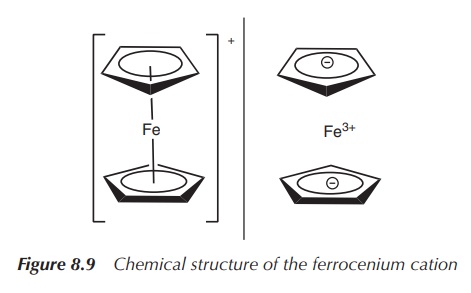
Ferrocene can easily undergo oxidation to the ferrocenium cation
in a one-electron oxidation process. The formed cation is fairly stable, and
the whole process is reversible. This redox potential, together with a change
in lipophilicity, is the main characteristic that makes ferrocene-based
compounds interesting for a variety of potential clinical applications,
especially the ones outlined in the following (Figure 8.9) .
Ferrocene and its derivatives as biosensors
Diabetes is a major health problem with hundreds of millions
sufferers worldwide. As part of the illness, diabetic patients have increased
glucose levels in their blood due to a lack of insulin or cells not reacting to
insulin. Insulin promotes the uptake of glucose into the cells. There are several
options to manage diabetes, but it is extremely crucial for the welfare of the
patients that the blood glucose levels are closely monitored. In order to
facilitate these regular measurements, a significant amount of research has
gone into the development of portable and easy-to-use devices. Modern blood
glucose monitors benefit from the technical advances of the so-called biosensor
research, an area where the majority of the biosensors are used.
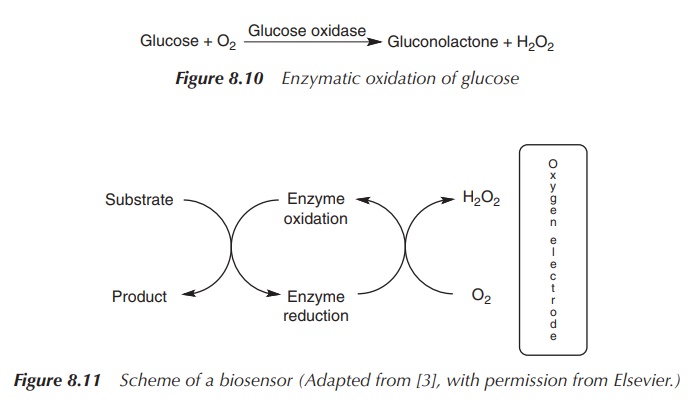
Biosensors are based on enzymes that contain
redox-active groups. This means that the redox group can change its redox state
as a result of a biochemical reaction. In nature, the enzymes glucose oxidase
(GOx) or glucose dehydrogenase (GDH) are used as biosensors for blood glucose
monitors. Typically, these enzymes accept electrons from the substrate, glucose
in this case, and oxidise it. The enzyme changes to its reduced state, which
normally deactivates the enzyme. In order to activate the enzyme again,
electrons are transferred and the enzyme is oxidised. GOx and GDH in their
reduced form transfer electrons to molecular oxygen, and hydrogen peroxide (H2O2)
is produced. Oxygen or peroxide electrodes can then be used to measure any
change of the substrate, which directly relates to the glucose levels present
in the sample. Unfortunately, this method has problems, as, for example,
molecular oxygen can be a limiting factor and a lack of oxygen can lead to
wrong readings (Figure 8.10).
Enzymes such as GOx are very specific to the substrate they
accept electrons from, that is, the substrates they oxidise, but they are more
flexible to the substrate they donate electrons to. Therefore, a variety of
inorganic redox-active compounds have been tested as so-called mediators.
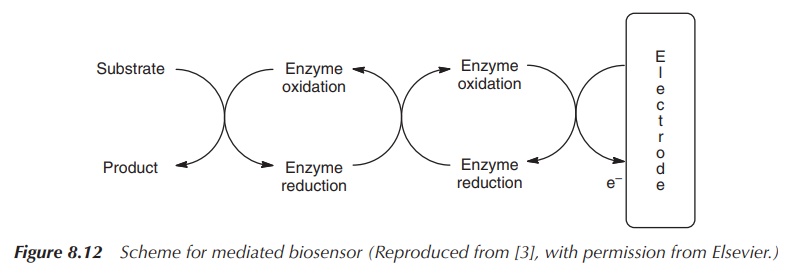
Mediators function by accepting electrons from the enzyme and thus oxidising
the enzyme to its active form. They shuttle electrons from the enzyme to the
electrode and are also called electron
sinks. Electrodes can measure any changes in the redox potential of these
mediators, and these changes can directly be related to the amount of glucose
present in the sample. This technology excludes the need for molecular oxygen
and problems connected to that (Figure 8.11) .
In 1984, the first ferrocene-based mediator in
conjunction with GOx was used as a biosensor for glucose. Derivatives of
ferrocene are still the most important examples for mediators in biosensors,
mainly due to their wide range of redox potential, which is independent of any
pH changes. Furthermore, the chemistry involved in synthesising these ferrocene
derivatives is well explored and fairly straight forward. Additionally, the
mediator must successfully compete with the natural mediator (molecular oxygen)
in order to ensure accurate readings. From the point of its application as
biosensor for blood glucose measurement, it is clear that ferrocene-based
mediators can be used only once. This is due to the fact that, whilst ferrocene
is relatively insoluble, the reduced form, the ferrocenium ion, is fairly
soluble. Mediators should be insoluble in order to lead to reproducible results
or, as in this case, can only be used once (Figure 8.12) [3, 4].
[Cp2Fe] → [Cp2Fe]+
+ e− (8.1)
Equation 8.1: Oxidation of ferrocene to the paramagnetic
ferrocenium ion.
Initially, ferrocene itself was used as mediator, but later on a
variety of its derivatives were tested for their redox potential in biosensors.
Some of these examples are shown below. This research has led to development of
the modern blood glucose analysers, which are only the size of a pen and highly
mobile devices. These devices use disposable strips and are simple to use
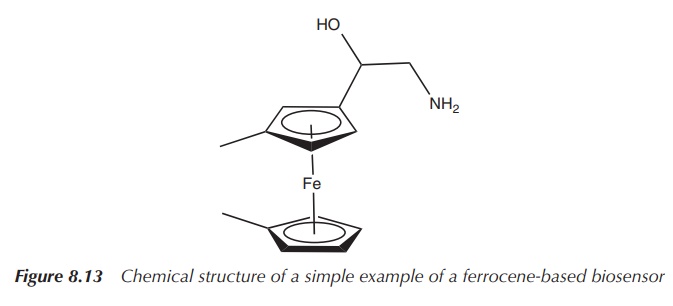
(Figure 8.13) .
Ferrocene derivatives as potential antimalarial agent
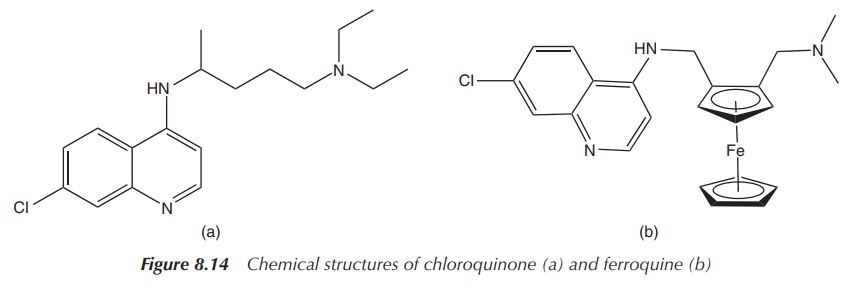
A variety of compounds containing the ferrocene group have been synthesised and tested for their clinical properties, especially as antimalarial and anticancer agents. In this context, especially the ferrocene-based analogue of chloroquine, ferroquine, has shown significant promise.
It successfully passed phase II clinical trials and is
awaiting results from field testing. Chloroquine is a well-known drug used in
the treatment of malaria caused by the parasite Plasmodium falciparum. Ferroquine is active against this parasite
as well. Even more exciting is the fact that it is also active against the
chloroquine-resistant strain of P.
falciparum. The changed biological activity might be due to the changed
lipophilicity and/or the redox action that is present after the introduction of
the ferrocene group (Figure 8.14) .
Ferrocifen – a new promising agent against breast cancer?
Ferrocene and its derivatives were, and still are, under intense
scrutiny as potential anticancer agents. Initially, a range of ferrocenium
salts was tested for their cytotoxic activity. The mode of action is still
unclear, but DNA, cell membrane and enzymes have been proposed as potential
targets. Ferrocenium salts are believed to gen-erate hydroxyl radicals under
physiological conditions. These may damage the DNA, possibly by oxidising the
DNA. Furthermore, it is believed that the cell membrane might be a target.
Research has shown that the counter-ion is crucial for the cytotoxic activity
as well as their aqueous solubility. Ferrocenium salts such as the picrate and
trichloroacetate derivates display good aqueous solubility and high cytotoxic
activity. As part of this research, ferrocene was also successfully bound to
polymers in order to improve their water solubility and therefore cytotoxic
activity .
Jaouen and coworkers substituted phenyl rings
in existing drugs and natural products by ferrocene groups in order to
introduce a redox-active metal group into these molecules and to change their
lipophilicity. A breakthrough was achieved when a phenyl group in tamoxifen, a
selective oestrogen receptor modulator (SERM) used as front-line treatment of
hormone-dependent breast cancer, was replaced by ferrocene. The active
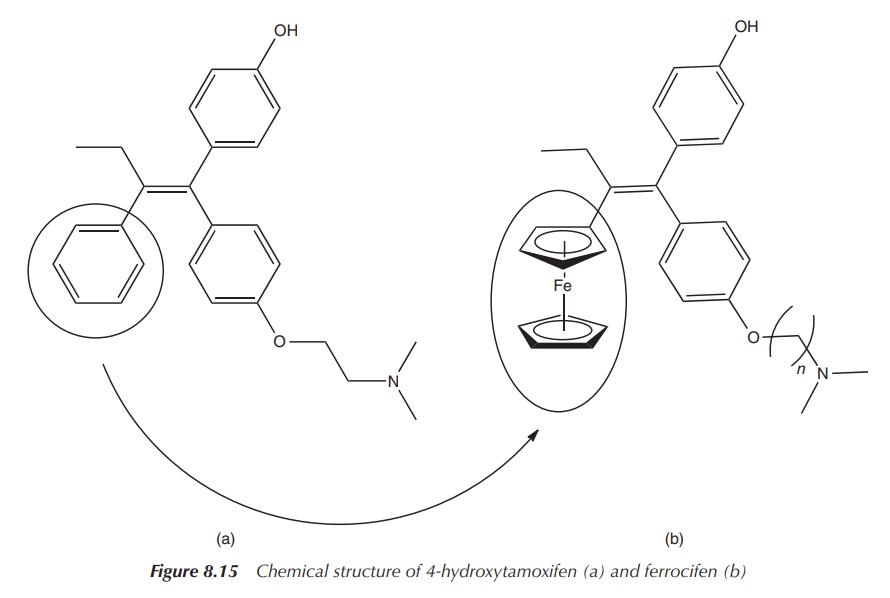
metabolite of tamoxifen is actually the hydroxylated form 4-hydroxytamoxifen,
which is highly active in the fight against oestrogen-dependent breast cancer.
Breast cancer can be divided into hormone-dependent (also called oestrogen-dependent, ER(+)), which is
characterised by the presence of an oestrogen receptor, and hormone-independent
(also called oestrogen-independent,
ER(−)) [2, 5].
Selective oestrogen receptor modulators are defined as a class of compounds that interact with the oestro-gen receptor. This interaction can happen in various
tissues leading to different actions.
The combination of tamoxifen derivatives with ferrocene was a
very successful approach, and has led to the development of a class of
compounds called ferrocifens. Whilst
around two-thirds of patients are diagnosed with ER(+) breast cancer and can be
treated with hormone therapy such as with SERMs, there is still an urgent need
to develop drugs to be used against ER(−) breast cancer. Preclinical studies
have shown that ferrocifen is active against the latter type of breast cancer
which is not susceptible to the treatment with tamoxifen (Figure 8.15) [2, 5].
The cytotoxic effect of tamoxifen results from the competitive
binding to the oestrogen receptor and repress-ing DNA transcription, which is
mediated by oestradiol. It is believed that ferrocifen follows the same mode of
action. Research has shown that the replacement of the phenyl group in
tamoxifen by ferrocene results in a reduced binding affinity to the receptor
(RBA, receptor binding affinity). Variation, and especially increase, of the
chain length has a negative effect on the RBA and also on the bioavailability.
The optimum chain length seems to be when n
= 4. It is also important to note that the Z-isomer
binds more strongly to the receptor. Very surprisingly, ferrocifen (with n = 4) showed also an antiproliferative
effect when tested on the oestrogen-independent cell line MDA-MB231, which does
not have oestrogen receptors and is not accessible for treatment with
tamoxifen. This means that there must be an additional mode of action that is
independent of the oestrogen receptor.
Replacing the ferrocenyl group by a ruthenium group resulted in
a drop of cytotoxic activity, indicating that the iron group is important. It
has been proposed that the additional mode of action of ferrocifen could rely
on the redox activation of the ferrocenyl group and the presence of reactive
oxygen species (ROS) .
These extremely promising results stimulated
further research in this area. Tamoxifen was coupled to a variety of known
metal-based compounds with potential anticancer activity, such as oxaliplatin,
titanocene dichloride and others. Oxaliplatin contains the so-called DACH–Pt group
(DACH, 1,2-diaminocyclohexane),
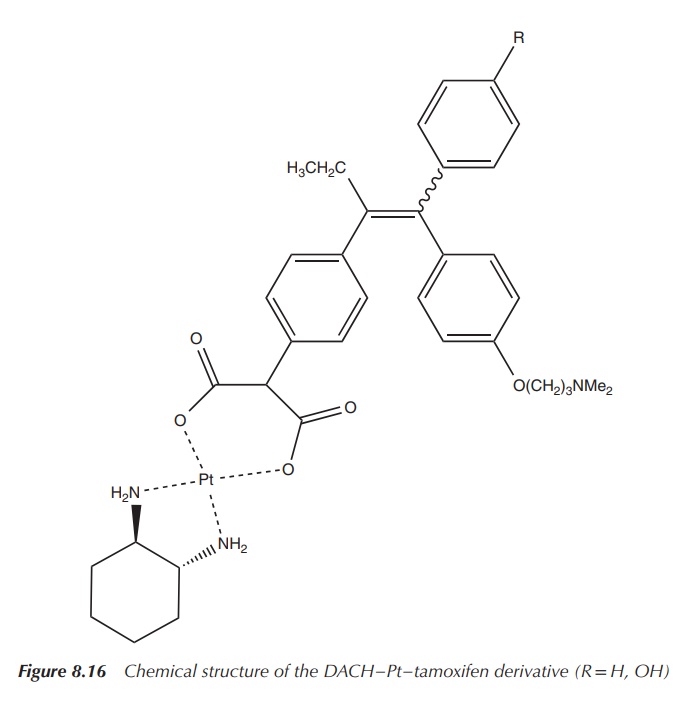
Oxaliplatin showed a cytotoxic effect of 6.3 μM when tested on the
oestrogen-dependent human breast cancer cell line MCF-7, whilst the
tamoxifen-vectorised derivatives (see Figure 8.16; R = H, 14 μM and R = OH, 4
μM) also presented an antiproliferative effect at a similar magnitude. Looking
in more detail, research shows that the derivative that contains the hydroxyl group
displays a higher RBA and also a better IC50 value. This shows that
the hydroxyl group (also present in the active metabolite of tamoxifen) is
important for the recognition by the oestrogen receptor. Nevertheless, the
vectorisation of DACH–Pt does not really result in a significant improvement in
comparison to oxaliplatin itself, and therefore this combination is not really
beneficial as an SERM for the fight against breast cancer .
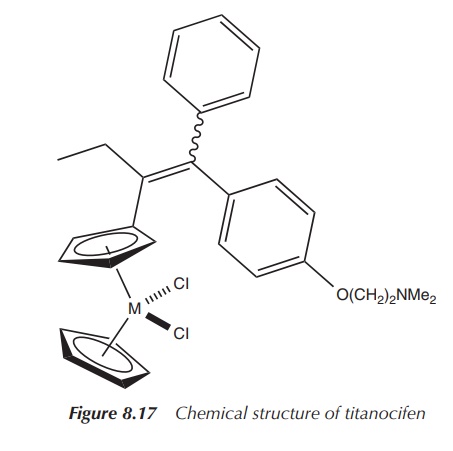
The titanocene-tamoxifen derivative has an RBA value of 8.5%,
which means it should recognise the oestro-gen receptor well. The results of
the cytotoxicity tests were very unexpected, where a proliferative effect was
observed. The estrogenic effect was comparable to that of oestrogen itself. It
is believed that this estrogenic effect is due to the titanium moiety and/or
its hydrolysis products (Figure 8.17) .
In order to bring ferrocifen into clinical
studies, it was important to find a suitable formulation. This is an area
notoriously difficult for metal-based drugs, and OH-ferrocifen finally entered
phase II clinical trials. A variety of pharmaceutical approaches have been
researched, including the use of nanoparticles, cyclodextrins and lipid
nanocapsules .
Related Topics
