The Clinical Use of Lanthanoids
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : The Clinical Use of Lanthanoids
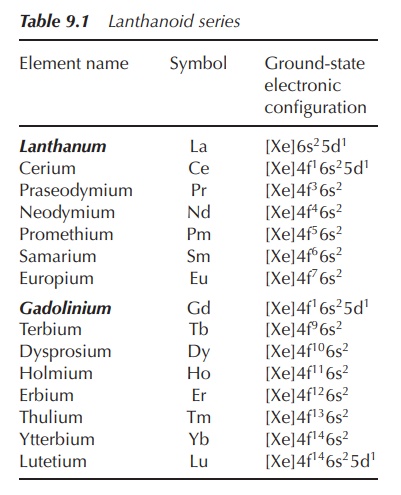
The lanthanoid series consists of 14 elements (cerium to lutetium) and they are also called f-block metals, as the valence shell of lanthanoids contains 4f orbitals.
The
Clinical Use of Lanthanoids
The lanthanoid series consists of 14 elements
(cerium to lutetium) and they are also called f-block metals, as the valence shell of lanthanoids contains 4f
orbitals. Lanthanoids are named after the element lanthanum (La), which itself
is a d-block metal. Nevertheless, because of its very similar chemical
behaviour, lanthanum is often also classified as a lanthanoid. The term rare earth metals describes the
lanthanoid series together with lanthanum (La), scandium (Sc) and yttrium (Y).
The lanthanoid series is often given the
symbol Ln and referred to the elements La–Lu.
The trivalent cation is the most stable oxidation state, as the
lanthanoids tend to lose three electrons (6s2 and 5d1)
because of their electronic configuration. The resulting trivalent cations
contain a xenon (Xe) core with regard to their electronic configuration with
the addition of varying numbers of 4f electrons. The 4f electrons can be found
closer to the nucleus, whilst the 5s2 and 5p6, which are
full sub-shells, shield them. Trivalent lanthanoids are often referred to as triple positive charged noble gases
(Table 9.1).
Related Topics
