The use of gadolinium salts as MRI contrast agents
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : The Clinical Use of Lanthanoids
The chemical element gadolinium has the chemical symbol Gd and atomic number 64. It is a silvery white metal, which is malleable and ductile. The dominant oxidation state of gadolinium salts is +III, with the resulting electronic configuration [Xe]4f7.
The
use of gadolinium salts as MRI contrast agents
The chemical element gadolinium has the chemical symbol Gd and
atomic number 64. It is a silvery white metal, which is malleable and ductile.
The dominant oxidation state of gadolinium salts is +III, with the resulting
electronic configuration [Xe]4f7.
Gadolinium is a relatively stable metal upon exposure to dry
air. Nevertheless, it tends to oxidise once it is exposed to moisture, as it
slowly reacts with water. Gadolinium is used in microwave applications and in
the manufacturing of compact discs and computer memory. Gadolinium is often
used in alloys. With as little as 1% gadolinium, the properties and workability
of iron and chromium improve.
Solutions containing gadolinium salts are used as CAs for MRI as
a clinical application. Using this method, it is possible to detect and observe
pathological and physiological alteration to living tissue. MRI used in
medicine uses the so-called relaxation properties of excited hydrogen nuclei
found in water and lipids in the human tissue. Relaxation refers to an effect
known in physics and chemistry, where there is a delay between the application
of an external stress to the system and its response. Within a strong magnetic
environment, which is produced in an MRI scanner, excited hydrogen nuclei show
different behaviours depending on their environment.
MRI can be carried out without any CAs, but
the use of a suitable CA can enhance the imaging properties. The basic idea is
that the water relaxation rates are altered in the presence of a CA, which
leads to additional and/or enhanced
information displayed in the images. In contrast to their role in the X-ray
imaging, the CAs themselves are not displayed in the images. Nowadays, around a
third of MRI scans are undertaken using CAs, and organ perfusions, kidney
clearance and changes in the blood–brain barrier can be detected (Figure 9.5).
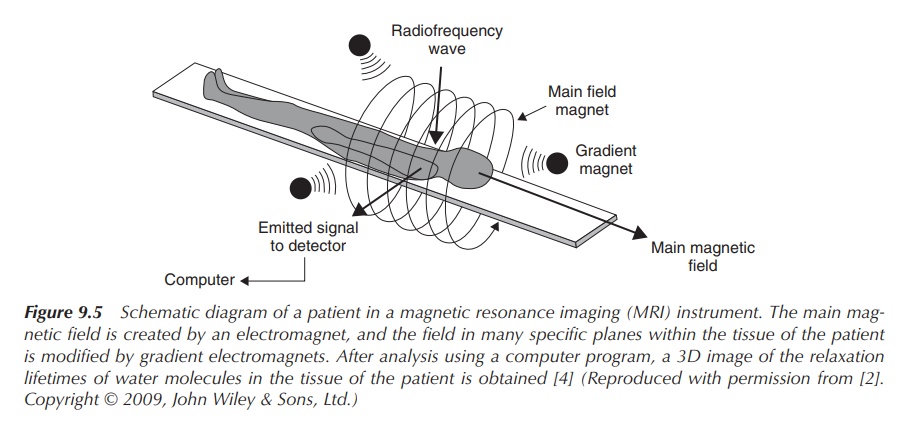
Figure 9.5 Schematic diagram of a patient in a magnetic resonance imaging (MRI) instrument. The main mag-netic field is created by an electromagnet, and the field in many specific planes within the tissue of the patient is modified by gradient electromagnets. After analysis using a computer program, a 3D image of the relaxation lifetimes of water molecules in the tissue of the patient is obtained (Reproduced with permission from . Copyright © 2009, John Wiley & Sons, Ltd.)
The trivalent cation Gd3+ is useful
as a CA for MRI, as it is a paramagnetic compound (see Chapter 7 Section 1.2
for the definition of paramagnetism) with the electronic configuration [Xe]4f7.
Gd3+ has seven unpaired elec-trons in its valence shell, which
endows it with the paramagnetic properties. Gd3+-containing CAs
affect the image quality of an MRI in two ways: (i) Paramagnetic Gd3+
complexes can coordinate water molecules and exchange them for water molecules
in their environment, which are coordinated to the metal centre. A typical Gd3+
complex is an eight-coordinated metal complex in which the ninth binding site
is available for the coordination of water. As previously discussed, the
typical coordination number for lanthanoid complexes
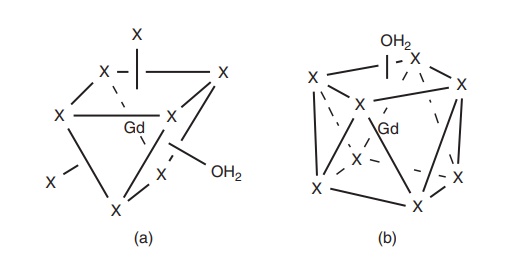
Figure 9.6 Coordination geometries of Gd3+ MRI CAs: (a) tricapped trigonal prism and (b)
monocapped square antiprism.
Depending on the structure of the octadentate ligand, which contributes eight
donor groups to Gd3+, denoted by X, and the location of the water
molecule, each geometry can exist in a number of isomeric forms (Reproduced with permission from . Copyright
© 2009, John Wiley & Sons, Ltd.)
Solutions of paramagnetic organic Gd3+ compounds are administered intravenously, and they enhance the images obtained by MRI. The trivalent ion itself has no physiological function in the human body and is highly toxic. Therefore, Gd3+ is administered as stable chelate in which the lanthanoid is chelated by an organic ligand and as a result exhibits a significantly lower toxicity.
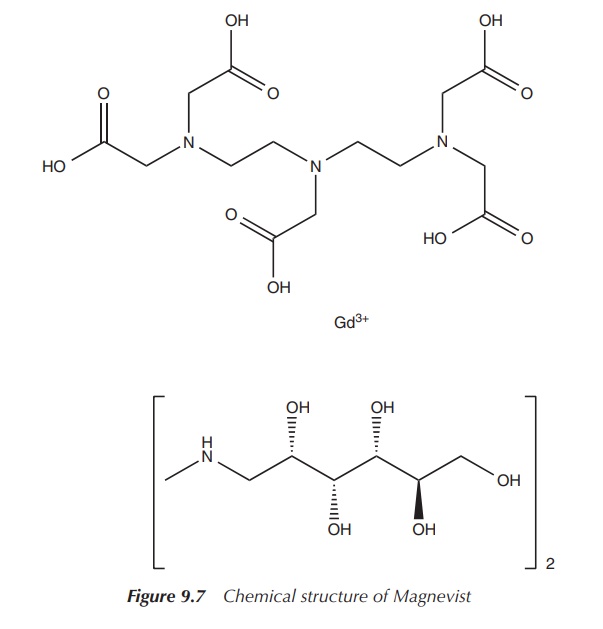
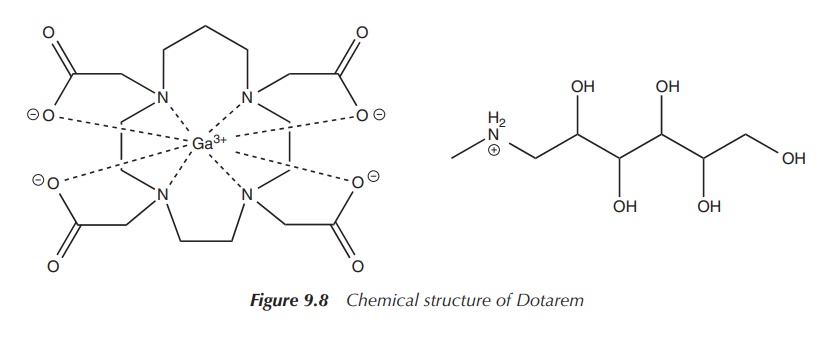
The most widespread clinical examples are
Magnevist™ and Dotarem™ (see Figures 9.7 and 9.8), both approved by the FDA for
their use as CAs in MRI. Gadolinium-containing chelates are around 50 times
less toxic than the ‘free’ Gd3+ in salts such as GdCl3.
Gadolinium-containing chelates are typically cleared from the blood plasma
within a few hours (half-life <2 h)
and excreted via the urine . In contrast, free Gd3+ ions remain in blood serum for significantly longer; only 2% is excreted after 7 days .
Related Topics
