The clinical use of lanthanum carbonate
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : The Clinical Use of Lanthanoids
The chemical element lanthanum has the symbol La and atomic number 57. It is a silvery white metal and represents the start of the lanthanoid series (Ln).
The
clinical use of lanthanum carbonate
The chemical element lanthanum has the symbol La and atomic
number 57. It is a silvery white metal and represents the start of the
lanthanoid series (Ln).
Lanthanum has two oxidation states, +II and +III, and the latter
is the more stable one. The electronic configuration of the resulting La3+
ion is [Xe]4f0. Lanthanum burns in the presence of air and forms
lan-thanum(III) oxide:
4La + 3O2 → 2La2O3
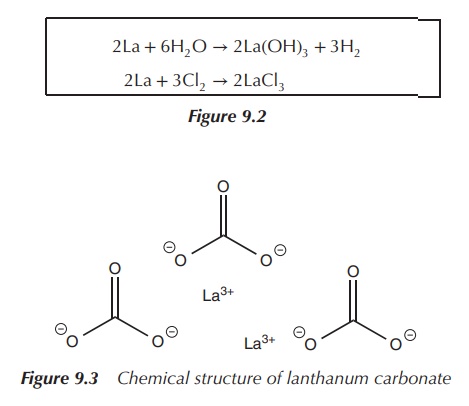
Lanthanum reacts with water with the formation of lanthanum
hydroxide owing to the electropositive nature of the metal. It also reacts with
halogens and forms the respective lanthanum halide salt.
Lanthanum carbonate La2(CO3)3
see Figure 9.2 is the only lanthanum salt approved for clinical use. It is used
in the managementof hyperphosphataemia, which is defined as high levels of
phosphate in the serum blood (Figure 9.3).
2La + 6H2O → 2La(OH)3 + 3H2
2La + 3Cl2 → 2LaCl3
Patients with end-stage renal failure (ESRF) often present high
levels of phosphate in the serum as a result of the failure of the kidneys. The
serum phosphate level of a healthy human is in the range 0.8–1.5 mmol/l ,
whereas this level is significantly increased in patients with kidney failure.
High phosphate levels are linked to a decrease of calcium serum levels and the
release of the so-called parathyroid hormone (PTH). This can manifest itself in
renal osteodystrophy, which can have severe pathological consequences such as
bone malfunction. Also, increased levels of PTH are observed, which can lead to
secondary calcification of muscles and vascular tissue. Nearly half of the
deaths of dialysis patients with ESRF are due to cardiac events.
The average phosphate intake ranges from 1000 to 1500 mg/day. In
a healthy human, phosphate is absorbed in the gastrointestinal (GI) tract and
excreted via the kidneys. In patients with ESRF, phosphate excretion via the
kidneys is reduced and therefore accumulates in the serum. A common treatment
option includes the binding of phosphate already in the GI tract before it can
enter the blood stream. The ideal phosphate binder should bind phosphate with high
affinity, should not be absorbed in the GI tract and should be excreted via the
faeces. Aluminium salts were used until the early 1980s as phosphate binders.
Aluminium phosphate is readily formed but not absorbed. Unfortunately, the
aluminium salt (aluminium hydroxide) itself is absorbed in the GI tract and has
been found to be toxic. Specific toxicity to the central nervous system (CNS)
was observed.
As an alternative treatment option, calcium salts were and still
are used as phosphate binders. Calcium salts, such as calcium acetate and
calcium carbonate, are very successful treatment options especially in patients
undergoing dialysis. The main issue is that calcium ions can be absorbed and
this can lead to hypercalcaemia, which is defined as high levels of serum
calcium ions. This can further increase the risk of tissue calcification and
cardiac events.
Further research has led to a variety of drugs, with sevelamer
hydrochloride being one of the most suc-cessful ones. Sevelamer is a hydrogel
containing cross-linked polyallylamine chains. The negatively charged phosphate
can be bound to the positively charged amine groups of the hydrogel in the
intestines and removed via the faeces.
Furthermore, lanthanum carbonate has been successfully studied
as a phosphate binding agent. Lanthanum carbonate fulfils the criteria for a
good phosphate binder as stipulated above: nontoxic, rapid binding of
phos-phate, not absorbed in the GI tract and easily excreted. A comparative
study of a variety of lanthanum salts showed that La2CO3⋅4H2O has the best phosphate binding
properties at a variety of pHs. This means that phosphate can be bound in the
stomach (very low pH) and the complex remains intact whilst travelling through
the GI tract where the pH is higher. Pharmacological studies have shown that
lanthanum carbonate is poorly absorbed when administered orally and that more
than 90% is excreted via the faeces. No specific toxicity has been observed
either. Lanthanum carbonate hydrate is marketed under the name Fosrenol and has
received approval in Europe and the United States for its clinical use in
patients with chronic renal failure .
Lanthanum carbonate hydrate is usually given with an initial
dose of 0.75–2.25 g of elemental lanthanum in divided doses with meal. The dose
needs to be reviewed every 2–3 weeks until a maintenance dose (1.5–3 g) is
achieved. Patients should be advised to chew the tablets before swallowing.
Most common side effects include disturbances of the GI tract, resulting in
diarrhoea and constipation .
Related Topics
