Titanocenes
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Organometallic Chemistry
Titanium, with chemical symbol Ti and atomic number 22, is a member of the d-block metals and belongs to group 4 of the periodic table of elements.
Titanocenes
Titanium, with chemical symbol Ti and atomic number 22, is a
member of the d-block metals and belongs to group 4 of the periodic table of
elements (Figure 8.18).
Titanium is a metal, which puzzles the bioinorganic scientists,
as it has no known native role in the human body (or any organism) despite it
is the ninth most abundant element in the earth’s crust . The metal titanium is
extremely corrosion-resistant and has a remarkable biocompatibility, which has
led to its clinical applications. These include endoprostheses of knees and
hips, dental implants, heart pacemakers and many more. Titanium complexes are
used for their catalytic properties, and there are complexes that interact with
biomolecules (this will be shown below). Nevertheless, there is no known function
of titanium as a native essential metal [6, 7].
The most stable oxidation state is Ti(IV), and most titanium
compounds with lower oxidation states are easily oxidised to Ti(IV). It is very
characteristic that these Ti(IV) species easily undergo hydrolysis and form
Ti—O bonds and fairly often polymeric structures . Titanium oxides and many
Ti(IV) compounds do not show high solubility in water and therefore have only
limited bioavailability. This might explain why titanium has no known
biological role despite its high natural abundance, and is not an essential
metal for any life form .
Titanium dioxide (TiO2) is a white solid with a low
aqueous solubility and is the natural occurring oxide of titanium present in
minerals such as rutile (composed mainly of TiO2). TiO2
is used as a white pigment in paints, food colouring, sunscreens and
toothpaste. For the purification of crude TiO2, the raw material is
reduced with carbon and then oxidised in the presence of chlorine. The
resulting titanium tetrachloride (TiCl4) is purified by distillation
and reacted with oxygen at around 2000 K to form TiO2 in its pure
form.
Current research into the medicinal applications of titanium complexes mainly focusses on their use as anticancer agents. It is important to note that these complexes are different from the above-described titanium alloys/metals or TiO2, which are highly insoluble in aqueous media. The titanium complexes described below contain functional groups that can be easily exchanged and/or interact with biomolecules in order to fulfil their medicinal role.
History of titanium-based anticancer agents: titanocene dichloride and budotitane
The success of cisplatin stimulated the search for further
metal-based anticancer drugs. Two titanium-based complexes, namely titanocene
dichloride (Cp2TiCl2) and budotitane (Ti(IV)(bzac)2(OEt)2,
bzac, 1-phenylbutane-1,3-dionate), were identified as promising drug candidates
and entered clinical trials . Both compounds contain labile groups in the cis
position analogous to cisplatin. Unfortunately, hydrolysis in titanium
compounds is faster than in cisplatin, and in these cases can lead to the
formation of hydroxo-bridge species and potentially to TiO2, which
is insoluble in water. This represents the main challenge for the clinical
application of this class of compounds (Figure 8.19) .
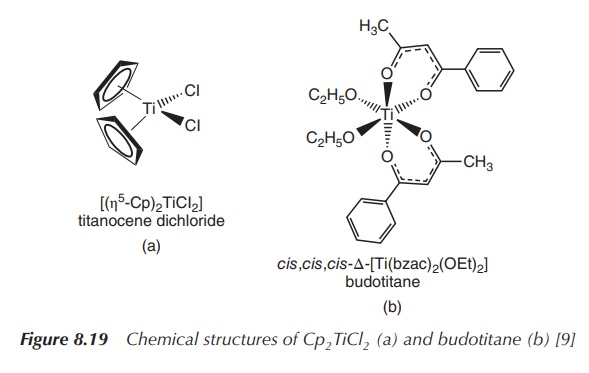
Cp2TiCl2 was first synthesised in 1954 and
is a red crystalline solid in its pure form. The Ti(IV) centre is coordinated
by two 5-Cp ligands and two chlorine ligands. Cp2TiCl2
can be synthesised by reacting titanium tetrachloride (TiCl4) with
NaCp . Compared to those of ferrocene, the planar cyclopentadienyl ligands are
coordinated to the titanium centre in a bent sandwich configuration .
Furthermore, Cp2TiCl2 is an exception to the 18 VEs rule,
as it has only 16 VEs, but it is still a stable complex. Each Cp−
ligand contributes six elec-trons, whilst each Cl− ligand
contributes two electrons. Ti(IV) has no VEs left, which makes a total of 16
VEs.
2NaC5H5 + TiCl4 → (C5H5)2TiCl2
+ 2NaCl
Compared to cisplatin, much less is known on the mode of action
of titanium-based drug candidates. In fact, the active species responsible for
the anticancer activity of Cp2TiCl2 in vivo has yet not been identified, nor has the coordination
mechanism to DNA or eventual repair processes. Studies have shown that titanium
will accumulate near phosphorus-rich areas, indicating a titanium–DNA
interaction takes place. Crystallo-graphic studies suggest that, within Cp2TiCl2,
the Cp groups sterically prevent the cross-linking of DNA. A direct comparison
between Cp2TiCl2 and cisplatin shows that their spectrum
of activity differs, leading to the conclusion that their mode of action must
also differ .
Current studies have confirmed the uptake of
Ti(IV) by transferrin and transport to the cancer cells. Within the cell, it is
believed that Ti(IV) may bind, in contrast to cisplatin, to the DNA backbone,
which means coordinating to the negatively charged phosphates. Generally, it is
believed that this coordination is not very strong .
Cp2TiCl2 showed very promising results in
preclinical studies. In vitro studies
have been carried out by Köpf-Maier on a variety of human cancers including
human lung and renal cell cancer carcinomas xenografted into mice. The results
were very encouraging, as a significant response of the tumour to the
chemotherapeutic agent was observed and Cp2TiCl2 seemed
to be an interesting candidate to bring into the clinic . The major challenge
for a clinical application of Cp2TiCl2 as an anticancer
agent is presented by its hydrolytic instability at pH below 5.5, where both chlorides
are lost. Even at neutral pH, research has shown that the Cp rings are lost
quickly, resulting in the formation of hydrolysed products which are water
insoluble . Therefore, it was necessary to develop a formulation before this
compound could be brought into clinical trials. For its use in clinical trials,
Cp2TiCl2 was formulated as a lyophilised powder in malate
buffer (pH = 3.2) or malic acid (pH = 3.5). Cp2TiCl2,
formulated as a water-soluble powder, has entered phase I and phase II clinical
trials [7]. Results from phase I clinical trials were used to establish the
maximum tolerable dose and to define the pharmacokinetic properties.
Dose-limiting toxicities have been identified, as reversible damage to the
liver and kidneys. Phase II clinical trials concentrated on the effect of Cp2TiCl2
treatment of renal cell cancer and metastatic breast cancer. Unfortunately, the
encouraging effect seen in preclinical studies could not be repeated in the
clinical studies. The tumours did not show a significant response to Cp2TiCl2,
and therefore clinical trials were discontinued .
Budotitane is an octahedral Ti(IV) complex that was developed as
a potential anticancer agent around the same time as the anticancer properties
of Cp2TiCl2 were discovered. Budotitane can be
synthesised by reacting TiCl4 with the appropriate diketonate in an
anhydrous solvent. Important structural features are the unsubstituted aromatic
rings, which positively contribute to the cytotoxic activity, and the
hydrolysable group. The latter group seems to be less important in regard to
the cytotoxicity of budotitane but crucial for formu-lation purposes as it
seems to determine the aqueous stability. Research has shown that the aqueous
stability increases with the hydrolysable group in the order: I− < Br− < Cl−
< F−
< OR−
(Figure 8.20) .
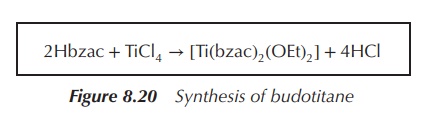
Figure 8.20 Synthesis of budotitane
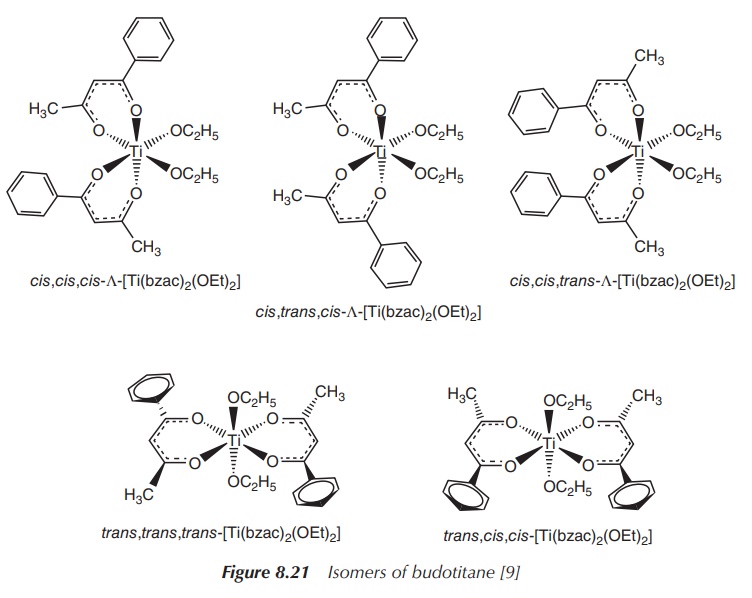
Another major drawback for the clinical use of budotitane is the
presence of isomers. There is the possi-bility of a cis or trans arrangement of
the —OC2H5 group, with the cis arrangement believed to be
the more stable one. Furthermore, the β-diketonato ligand is not symmetrical.
This means that there are eight possible arrangements in this octahedral
scheme. In solution, there is a mixture of isomers and it is difficult to
isolate the isomers. So far, it is not known which isomer exhibits the
anticancer activity (Figure 8.21) .
Preclinical studies using human xenografts in
nude mice highlighted the potential of budotitane as novel chemotherapeutic
agent in 1984. Budotitane showed cytotoxic activity comparable to cisplatin.
Keppler et al. intensively studied
its antitumour activity in a variety of animal models and found specific
activity against Ehrlich ascetic tumour and colon cancer. Budotitane, similar
to Cp2TiCl2, is prone to hydrolysis in conjunction with a
low aqueous solubility. Therefore, significant research went into the
development of an appropriate for-mulation . Several clinical studies were
performed using cremophor EL as a nonionic surfactant, which has been used as a
vehicle for the solubilisation of a wide variety of hydrophobic drugs
(cremophor). Furthermore, 1,2-polyethyleneglycol has been added to encourage
the coprecipitation of the drug. All three ingredients (budotitane, cremophor
EL and 1,2-polyethyleneglycol) are dissolved in anhydrous ethanol and mixed,
and the solvent is then evaporated off. Using this procedure, micelles
containing 100–200 mg/100 ml of active pharmaceutical ingredient are formed,
which are stable for a few hours. Budotitane went through several phase I and
phase II clinical trials. Budotitane was administered as intravenous (IV)
infusion twice a week to patients with solid tumours refractory to previous
treatments. Cardiac arrhythmia was identified as the dose-limiting
2Hbzac + TiCl4 → [Ti(bzac)2(OEt)2] + 4HCl
side effect. Unfortunately, problems with the formulation, such
as the existence of isomers and the difficulty in analysing and characterising
the loaded micelles, led to a discontinuation of the clinical trials .
Poor water solubility and fast hydrolysis are the main problems
for the use of titanocene or titanium-based compounds as anticancer agents.
Nevertheless, this intensive research in the 1980s and 1990s has encouraged
further research as described in the following.
Further developments of titanocenes as potential anticancer agents
As mentioned, poor aqueous solubility and instability in water
are the main problems that restrict titanocenes in clinical applications.
McGowan and coworkers renewed the interest in research in this area with their
elegant synthesis of ring-substituted cationic titanocene dichloride
derivatives. The idea was based on the introduction of charges in order to
improve the aqueous solubility. Indeed, the resulting cationic titanocenes are
more water-soluble and show a significant cytotoxic activity especially against
cisplatin-resistant ovarian cancer (Figure 8.22) .
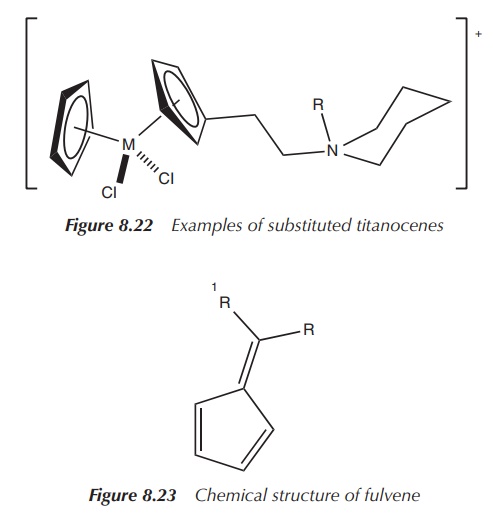
Following on from these results, Tacke and coworkers based their
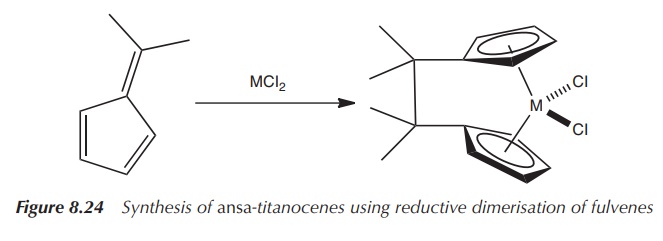
research on novel synthetic methods starting from substituted fulvenes. A
fulvenes is an organic molecule with the chemical formula C6H6,
which consists of a five-membered ring system and can be used to easily
introduce functional groups into titanocenes (Figure 8.23) .
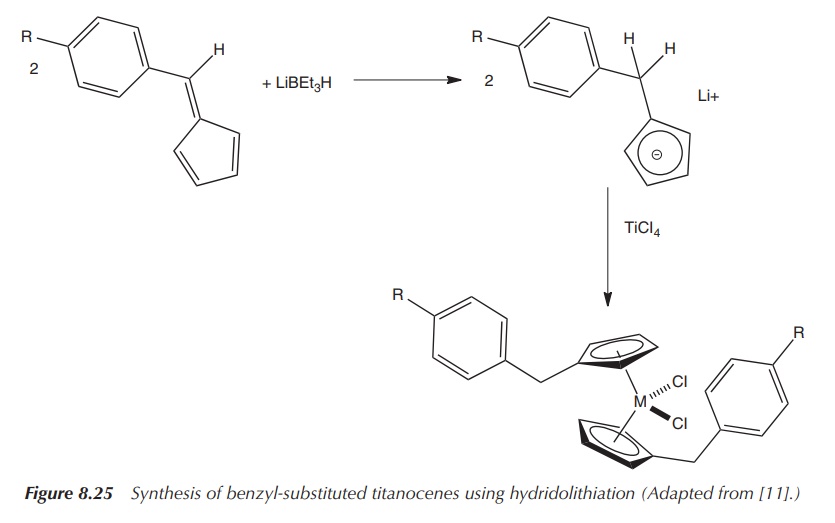
Reaction of substituted fulvenes with titanium
dihalides via a reductive dimerisation process leads to the so-called ansa-titanocenes, which are normally
used as catalysts. These ansa-titanocenes
are characterised by a carbon–carbon bridge between the cyclopentadienyl (Cp)
rings, which restricts the geometry of the Cp
In vitro
testing showed that they exhibit a moderate cytotoxic activity, which is
similar or better than Cp2TiCl2 when tested against a model
of renal cell cancer (Figure 8.24) .
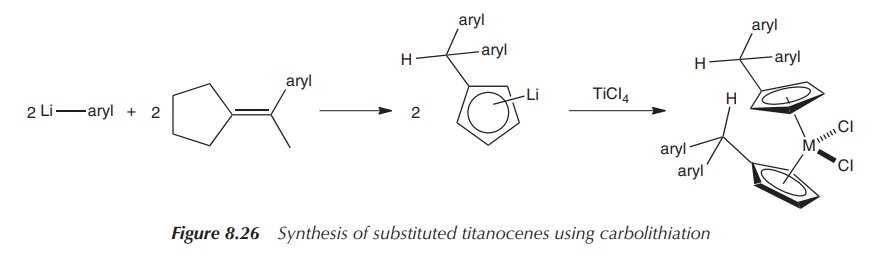
The reaction of substituted fulvenes with superhydride (LiBEt3H)
in a so-called hydridolithiation reaction or the reaction with an organolithium
compound in a carbolithiation reaction followed by transmetallation with
titanium tetrachloride allows access to a variety of substituted titanocenes.
Hydridolithiation allows access to benzyl-bridged titanocenes, whilst
titanocenes obtained via carbolithiation typically contain more functional
groups. This can be of advantage, for example, for the introduction of groups
that can be eas-ily ionised in order to improve the water solubility of these
titanocenes, but can also be a disadvantage as additional stereocentres are
potentially introduced (Figures 8.25 and 8.26).
These titanocenes have been intensively studied with regard to their potential anticancer activity. It has been shown that some of these compounds are active against a variety of human cancer types. The so-called titanocene Y has been the most intensively studied titanocene of this series. It has shown good activity against a model of renal cell cancer as well as other human cancer cell lines in a variety of in vitro experiments (Figure 8.27).
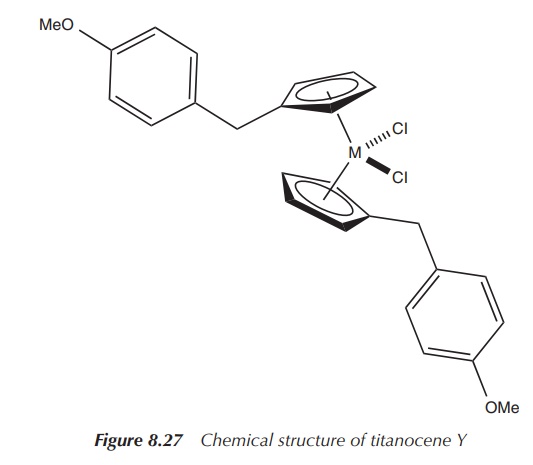
In general, titanocenes obtained via these
methods reached IC50 values in the low micromolar range when tested
against a renal cell cancer model. This represents an up to 2000-fold
improvement compared to Cp2TiCl2. Nevertheless, the main
problems are the potential presence of stereocentres and still a poor aqueous
solubility. Further research has been carried out with the aim to replace the
chloride ligands by other groups. This includes the incorporation of chelating
ligands similar to the research undertaken for cisplatin. Nevertheless, this research
did not result in any major improvement with regard to the cytotoxic activity.
Also, some initial formulation studies have been carried out with only limited
success. The mode of action of these titanocenes is so far not clear. It is
believed that they coordinate to DNA via its phosphate backbone. It has also
been shown that they can use transferrin as a transporter molecule into the
cancer cell .
Related Topics
