General Anaesthetics
| Home | | Pharmacology |Chapter: Essential pharmacology : General Anaesthetics
General anaesthetics (GAs) are drugs which produce reversible loss of all sensation and consciousness.
GENERAL ANAESTHETICS
General anaesthetics (GAs) are drugs which produce reversible
loss of all sensation and consciousness. The cardinal features of general
anaesthesia are:
·
Loss of all sensation, especially pain
·
Sleep (unconsciousness) and amnesia
·
Immobility and muscle relaxation
·
Abolition of somatic and autonomic reflexes.
In the modern practice of balanced anaesthesia, these modalities
are achieved by using combination of inhaled and i.v. drugs, each drug for a specific
purpose; anaesthesia has developed as a highly specialized science in itself.
History
Before the middle of
19th century a number of agents like alcohol,
opium, cannabis, or even concussion and asphyxia were used to obtund surgical
pain, but operations were horrible ordeals. Horace Wells, a dentist, picked up
the idea of using nitrous oxide (N2O)
from a demonstration of laughing gas in 1844. However, he often failed to
relieve dental pain completely and the use of N2O had to wait till
other advances were made. Morton, a dentist and medical student at Boston,
after experimenting on animals, gave a demonstration of ether anaesthesia in 1846, and it soon became very popular. Chloroform was used by Simpson in
Britain for obstetrical purpose in
1847, and despite its toxic potential, it became a very popular surgical
anaesthetic. Cyclopropane was introduced
in 1929, but the new generation of anaesthetics was heralded by halothane in 1956. The first i.v.
anaesthetic thiopentone was
introduced in 1935.
Mechanism Of General Anaesthesia
The mechanism of action
of GAs is not precisely known. A wide variety of chemical agents produce general
anaesthesia. Therefore, GA action had been related to some common physicochemical
property of the drugs. Mayer and Overton (1901) pointed out a direct
parallelism between lipid/water partition coefficient of the GAs and their
anaesthetic potency.
Minimal Alveolar Concentration (MAC) is the lowest concentration of the anaesthetic in pulmonary
alveoli needed to produce immobility in response to a painful stimulus (surgical
incision) in 50% individuals. It is accepted as a valid measure of potency of
inhalational GAs because it remains fairly constant for a given species even
under varying conditions.
The MAC of a number of
GAs shows excellent correlation with their oil/gas partition coefficient.
However, this only reflects capacity of the anaesthetic to enter into CNS and
attain sufficient concentration in the neuronal membrane, but not the mechanism
by which anaesthesia is produced.
Recent evidence
favours a direct interaction of the GA molecules with hydrophobic domains of
membrane proteins or the lipidprotein interface.
It has now been realised that different anaesthetics may be acting
through different molecular mechanisms, and various components of the
anaesthetic state involve action at discrete loci in the cerebrospinal axis.
The principal locus of causation of unconsciousness appears to be in the
thalamus or reticular activating system, amnesia may result from action in
hippocampus, while spinal cord is the likely seat of immobility on surgical stimulation.
Recent findings show
that ligand gated ion channels (but not voltage sensitive ion channels) are the
major targets of anaesthetic action. The GABAA receptor gated Cl¯
channel is the most important of these. Many inhalational anaesthetics, barbiturates,
benzodiazepines and propofol potentiate the action of inhibitory transmitter
GABA to open Cl¯ channels. Each of the above anaesthetics appears to interact
with its own specific binding site on the GABAA receptor Cl¯ channel
complex, but none binds to the GABA binding site as such; though some inhaled anaesthetics
and barbiturates (but not benzodiazepines) can directly activate Cl– channels.
Action of glycine (another inhibitory transmitter which also activates Cl¯ channels)
in the spinal cord and medulla is augmented by barbiturates, propofol and many inhalational
anaesthetics. This action may block responsiveness to painful stimuli resulting
in immobility of the anaesthetic state. Certain fluorinated anaesthetics and
barbiturates, in addition, inhibit the neuronal cation channel gated by
nicotinic cholinergic receptor which may mediate analgesia and amnesia.
On the other hand, N2O
and ketamine do not affect GABA or glycine gated Cl¯ channels. Rather they
selectively inhibit the excitatory NMDA type of glutamate receptor. This
receptor gates mainly Ca2+ selective cation channels in the neurones,
inhibition of which appears to be the primary mechanism of anaesthetic action
of ketamine as well as N2O. The volatile anaesthetics have little
action on this receptor.
Neuronal
hyperpolarization caused by GAs has been ascribed to activation of a specific
type of K+ channels, while inhibition of transmitter release from presynaptic
neurones has been related to interaction with certain critical synaptic proteins.
Thus, different facets of anaesthetic action may have distinct neuronal basis,
as opposed to the earlier belief of a global neuronal depression.
Unlike local
anaesthetics which act primarily by blocking axonal conduction, the GAs appear
to act by depressing synaptic transmission.
Stages Of Anaesthesia
GAs cause an irregularly
descending depression of the CNS, i.e. the higher functions are lost first and
progressively lower areas of the brain are involved, but in the spinal cord
lower segments are affected somewhat earlier than the higher segments. The vital
centres located in the medulla are paralysed the last as the depth of
anaesthesia increases. Guedel (1920) described four stages with ether anaesthesia, dividing the stage
into 4 planes. These clearcut stages are not seen nowadays with the use of faster
acting GAs, premedication and employment of many drugs together. The precise
sequence of events differs somewhat with anaesthetics other than ether.
However, ether continues to be used in India and description of these stages
still serves to define the effects of light and deep anaesthesia. Important
features of different stages are depicted in Fig. 27.1.
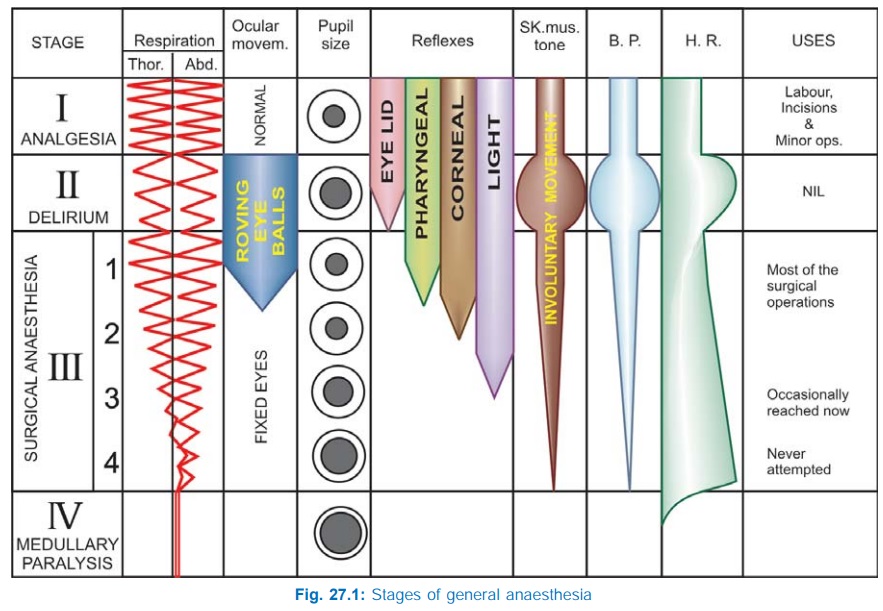
I. Stage Of Analgesia
Starts from beginning of anaesthetic inhalation and lasts upto the
loss of consciousness. Pain is progressively abolished. Patient remains conscious,
can hear and see, and feels a dream like state; amnesia develops by the end of
this stage. Reflexes and respiration remain normal.
Though some minor
operations can be carried out during this stage, it is rather difficult to
maintain—use is limited to short procedures.
II. Stage Of
Delirium
From loss of consciousness
to beginning of regular respiration. Apparent excitement is seen—patient may
shout, struggle and hold his breath; muscle tone increases, jaws are tightly
closed, breathing is jerky; vomiting, involuntary micturition or defecation may
occur. Heart rate and BP may rise and pupils dilate due to sympathetic
stimulation.
No stimulus should be applied or operative procedure carried out
during this stage. This stage is inconspicuous in modern anaesthesia.
III. Surgical Anaesthesia
Extends from onset of regular respiration
to cessation of spontaneous breathing. This has been divided into 4 planes
which may be distinguished as:
Plane 1 Roving eyeballs. This plane ends when eyes
become fixed.
Plane 2 Loss of corneal and laryngeal reflexes.
Plane 3 Pupil starts dilating and light reflex is lost.
Plane 4 Intercostal paralysis,
shallow abdominal respiration, dilated pupil.
As anaesthesia passes
to deeper planes, progressively—muscle tone decreases, BP falls, HR increases
with weak pulse, respiration decreases in depth and later in frequency also—
thoracic lagging behind abdominal.
IV. Medullary Paralysis
Cessation of breathing to failure of circulation and death. Pupil is
widely dilated, muscles are totally flabby, pulse is thready or imperceptible
and BP is very low.
Many of the above
indices have been robbed by the use of atropine (pupillary, heart rate), morphine
(respiration, pupillary), muscle relaxants (muscle tone, respiration, eye
movements, reflexes) etc. and the modern anaesthetist has to depend on several
other observations to gauge the depth of anaesthesia.
a)
If eyelash reflex is present and patient is
making swallowing movements—stage II has not been reached.
b) Loss of response to
painful stimulus (e.g. pressure on the upper nasal border of orbit)
— stage III has been reached.
c)
Incision of the skin causes reflex increase in
respiration, BP rise or other effects; insertion of endotracheal tube is resisted
and induces coughing, vomiting, laryngospasm; tears appear in eye; passive
inflation of lungs is resisted—anaesthesia is light.
d) Fall in BP, cardiac
and respiratory depression are signs of deep anaesthesia.
In the present day practice
anaesthesia is generally kept light; adequate analgesia, amnesia and muscle
relaxation are produced by the use of intravenous drugs. Premedication with CNS
depressants and opioids or their concurrent use lowers MAC of the inhaled
anaesthetic. When a combination of two inhalational anaesthetics (e.g. N2O
+ isoflurane) is used, their MACs are additive: lower concentration of each is
required. The doseresponse relationship of inhaled anaesthetics is very steep;
just 10% higher concentration (1.1 MAC) immobilizes >90% subjects.
Concentrations of inhalational anaesthetics exceeding 1.2 MAC are rarely used,
and 2–3 MAC is often lethal.
Pharmacokinetics Of Inhalational Anaesthetics
Inhalational anaesthetics are gases or vapours that diffuse
rapidly across pulmonary alveoli and tissue barriers. The depth of anaesthesia
depends on the potency of the agent (MAC is an index of potency) and its
partial pressure (PP) in the brain, while induction and recovery depend on the
rate of change of PP in the brain. Transfer of the anaesthetic between lung and
brain depends on a series of tension gradients which may be summarized as—
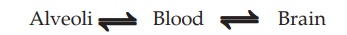
Factors affecting the PP of anaesthetic attained in the brain
are—
1. PP of Anaesthetic In The Inspired Gas
This is proportional to its
concentration in the inspired gas mixture. Higher the inspired tension more
anaesthetic will be transferred to the blood. Thus, induction can be hastened
by administering the GA at high concentration in the beginning.
2. Pulmonary
Ventilation
It governs delivery of the GA to the alveoli. Hyperventilation will
bring in more anaesthetic per minute and respiratory depression will have the
opposite effect. Influence of minute volume on rate of induction is greatest in
the case of agents which have high blood solubility because their PP in blood
takes a long time to approach the PP in alveoli. However, it does not affect
the terminal depth of anaesthesia attained with any concentration of a GA.
3. Alveolar
Exchange
The GAs diffuse freely across alveoli, but if alveolar ventilation
and perfusion are mismatched (as occurs in emphysema and other lung diseases)
the attainment of equilibrium between alveoli and blood is delayed: well
perfused alveoli may not be well ventilated—blood draining these alveoli carries
less anaesthetic and dilutes the blood coming from well ventilated alveoli.
Induction and recovery both are slowed.
4. Solubility Of Anaesthetic In Blood
This is the most important property
determining induction and recovery. Large amount of an anaesthetic that is highly
soluble in blood (ether) must dissolve before its PP is raised. The rise as
well as fall of PP in blood and consequently induction as well as recovery are
slow. Drugs with low blood solubility, e.g. N2O, sevoflurane,
desflurane induce quickly.
Blood: gas partition
coefficient (λ) given by the ratio
of the concentration of the anaesthetic in blood to that in the gas phase at
equilibrium is the index of solubility of the GA in blood.
5. Solubility
Of Anaesthetic In Tissues
Relative solubility of the anaesthetic in blood and
tissue determines its concentration in that tissue at equilibrium. Most of GAs
are equally soluble in lean tissues as in blood, but more soluble in fatty
tissue. Anaesthetics with higher lipid solubility (halothane) continue to enter
adipose tissue for hours and also leave it slowly. The concentration of these
agents is much higher in white matter than in grey matter.
6. Cerebral Blood
Flow
Brain is a highly perfused organ; as such GAs are quickly
delivered to it. This can be hastened by CO2 inhalation which causes
cerebral vasodilatation—induction and recovery are accelerated. Carbon dioxide
stimulates respiration and this also speeds up the transport.
Elimination
When anaesthetic
administration is discontinued, gradients
are reversed and the channel of absorption (pulmonary epithelium) becomes the
channel of elimination. All inhaled anaesthetics are mainly eliminated through
lungs. The same factors which govern induction also govern recovery.
Anaesthetics, in general, continue to enter and persist for long periods in
adipose tissue because of their high lipid solubility and low blood flow to
fatty tissues. Muscles occupy an intermediate position between brain and adipose
tissue. Most GAs are eliminated unchanged. Metabolism is significant only for
halothane which is >20% metabolized in liver. Others are practically not
metabolized. Recovery may be delayed after prolonged anaesthesia, especially in
case of more lipidsoluble anaesthetics (halothane, isoflurane), because large
quantities of the anaesthetic have entered the muscle and fat, from which it is
released slowly into blood.
Second Gas Effect And Diffusion Hypoxia
In the initial part of
induction, diffusion gradient from alveoli to blood is high and larger quantity
of anaesthetic is entering blood. If the inhaled concentration of anaesthetic
is high, substantial loss of alveolar gas volume will occur and the gas mixture
will be sucked in, independent of ventilatory exchange—gas flow will be higher
than tidal volume. This is significant only with N2O, since it is
given at 70–80% concentration; though it has low solubility in blood, about 1
litre/min of N2O enters blood in the first few minutes—gas flow is 1
litre/min higher than minute volume. If another potent anaesthetic, e.g. halothane
(1–2%) is being given at the same time, it also will be delivered to blood at a
rate 1 litre/min higher than minute volume and induction will be faster—second gas effect.
The reverse occurs when N2O is discontinued after
prolonged anaesthesia—N2O having low blood solubility rapidly
diffuses into alveoli and dilutes the alveolar air—PP of oxygen in alveoli is
reduced. The resulting hypoxia, called diffusion
hypoxia, is not of much consequence if
cardiopulmonary reserve is normal, but may be dangerous if it is low. This
can be prevented by continuing 100% O 2 inhalation for a few minutes
after discontinuing N2O, instead of straight away switching over to
air. Diffusion hypoxia is not significant with other anaesthetics because they are
administered at low concentrations (0.2–4%) and cannot dilute alveolar air by
more than 1–2%.
Techniques Of Inhalation Of Anaesthetics
Different techniques are used according to facility available,
agent used, condition of the patient, type and duration of operation.
1. Open Drop Method
Liquid anaesthetic is poured over a mask with gause
and its vapour is inhaled with air. A lot of anaesthetic vapour escapes in the
surroundings and the concentration of anaesthetic breathed by the patient
cannot be determined. It is wasteful—can be used only for cheap anaesthetics.
Some rebreathing does occur in this method. However, it is simple and requires
no special apparatus. Ether is the only agent used by this method, especially
in children.
2. Through Anaesthetic Machines
Use is made of gas cylinders, specialized
graduated vaporisers, flow meters, unidirectional valves, corrugated rubber
tubing and reservoir bag.
The gases are delivered to the patient through a tightly fitting
face mask or endotracheal tube. Administration of the anaesthetic can be more
precisely controlled and in many situations its concentration determined.
Respiration can be controlled and assisted by the anaesthetist.
a) Open System The exhaled gases are allowed to escape through a valve and fresh anaesthetic
mixture is drawn in each time. No rebreathing is allowed—flow rates are
high—more drug is consumed. However, inhaled O2 and anaesthetic
concentration can be accurately delivered.
b) Closed System The patient rebreaths the exhaled gas mixture after it has circulated through
sodalime which absorbs CO2. Only as much O2 and
anaesthetic as have been taken up by the patient are added to the circuit. The
flow rates are low; especially useful for expensive and explosive agents
(little anaesthetic escapes in the surrounding air) e.g. halothane, enflurane,
isoflurane. However, control of inhaled anaesthetic concentration is difficult.
c) Semiclosed
System Partial rebreathing is
allowed through a partially
closed valve. Conditions are intermediate with moderate flow rates.
Properties Of An Ideal Anaesthetic
A) For The
Patient
It should be pleasant,
nonirritating, should not cause nausea or vomiting. Induction and recovery
should be fast with no after effects.
B) For The
Surgeon
It should provide
adequate analgesia, immobility
and muscle relaxation. It should be noninflammable and nonexplosive so that
cautery may be used.
C) For The Anaesthetist
Its administration should be easy, controllable
and versatile.
Margin of safety should be wide—no fall in BP.
Heart, liver and other organs should not be affected.
It should be potent so that low concentrations are needed and
oxygenation of the patient does not suffer.
Rapid adjustments in depth of anaesthesia should be possible.
It should be cheap, stable and easily stored. It should not
react with rubber tubing or soda lime.
The important physical and anaesthetic properties of inhalational
anaesthetics are presented in Table 27.1.
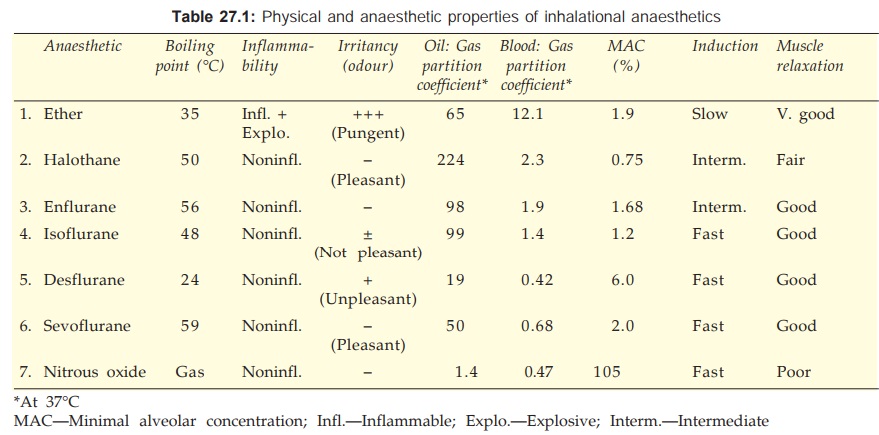
Classification
Inhalational
Gas
Nitrous oxide
Volatile liquids
Ether
Halothane
Enflurane
Isoflurane
Desflurane
Sevoflurane
Intravenous
Inducing agents
Thiopentone sod.
Methohexitone sod.
Propofol
Slower acting drugs
Benzodiazepines
Diazepam
Lorazepam
Etomidate
Midazolam
Dissociative anaesthesia
Ketamine
Opioid analgesia
Fentanyl
Cyclopropane, trichloroethylene
and methoxyflurane are no longer used.
Inhalational Anaesthetics
Nitrous oxide (N2O)
It is a colourless, odourless, heavier than air, noninflammable
gas supplied under pressure in steel cylinders. It is nonirritating, but low potency
anaesthetic; unconsciousness cannot be produced in all individuals without
concomitant hypoxia: MAC is 105% implying that even pure N2O cannot
produce adequate anaesthesia at 1 atmosphere pressure. Patients maintained on
70% N2O + 30% O2 along with muscle relaxants often recall
the events during anaesthesia, but some lose awareness completely.
Nitrous oxide is a
good analgesic; even 20% produces analgesia equivalent to that produced by
conventional doses of morphine. It is a poor muscle relaxant; neuromuscular blockers
are often required. Onset of N2O action is quick and smooth (but
thiopentone is often used for induction), recovery is rapid: both because of
its low blood solubility. Second gas effect and diffusion hypoxia occur with N2O
only. Post-anaesthetic nausea is not marked.
Nitrous oxide is
generally used as a carrier and adjuvant to other anaesthetics. A mixture of
70% N2O + 25–30% O2 + 0.2–2% another potent anaesthetic
is employed for most surgical procedures. In this way concentration of the
other anaesthetic can be reduced to 1/3 for the same level of anaesthesia.
Because N2O has little effect on respiration, heart and BP:
breathing and circulation are better maintained with the mixture than with the
potent anaesthetic given alone in full doses. However, N2O can
expand pneumothorax and other abnormal air pockets in the body.
As the sole agent, N2O
(50%) has been used with O2 for dental and obstetric analgesia. It
is nontoxic to liver, kidney and brain. Metabolism of N2O does not
occur; it is quickly removed from body by lungs. It is cheap and very commonly
used.
Ether (Diethyl ether)
It is a highly
volatile liquid, produces
irritating vapours which are inflammable and explosive.
(C2H5 — O —
C2H5)
Ether is a potent anaesthetic, produces good analgesia and
marked muscle relaxation by reducing ACh output from motor nerve endings —dose
of competitive neuromuscular blockers should be reduced to about 1/3.
It is highly soluble in blood—induction is prolonged and
unpleasant with struggling, breath-holding, salivation and marked respiratory
secretions (atropine must be given as premedication to prevent the patient from
drowning in his own secretions). Recovery is slow; post-anaesthetic nausea, vomiting
and retching are marked.
BP and respiration are generally well maintained because of
reflex stimulation and high sympathetic tone. It does not sensitize the heart
to Adr, and is not hepatotoxic.
Ether is not used now in developed countries because of its
unpleasant and inflammable properties. However, it is still used in developing
countries, particularly in peripheral areas because it is—cheap, can be given
by open drop method (though congestion of eye, soreness of trachea and ether burns
on face can occur) without the need for any equipment, and is relatively safe
even in inexperienced hands.
Halothane (FLUOTHANE)
It is a volatile liquid with sweet
odour, nonirritant and noninflammable. Solubility in blood is intermediate— induction
is reasonably quick and pleasant.
It is a potent anaesthetic—precise control of administered
concentration is essential. For induction 24% and for maintenance 0.5–1% is
delivered by the use of a special vaporizer. It is not a good analgesic or
muscle relaxant; however, it potentiates competitive neuromuscular blockers.
Halothane causes direct
depression of myocardial contractility by reducing intracellular Ca2+
concentration. Moreover, sympathetic activity fails to increase (as occurs with
ether). Cardiac output is reduced with deepening anaesthesia. BP starts falling
early and parallels the depth. Many vascular beds dilate but total peripheral
resistance is not significantly reduced. Heart rate is reduced by vagal
stimulation, direct depression of SA nodal automaticity and lack of
baroreceptor activation even when BP falls. It tends to sensitize the heart to
the arrhythmogenic action of Adr. The electrophysiological effects are
conducive to reentry—tachyarrhythmias occur occasionally.
Halothane causes relatively
greater depression of respiration; breathing is shallow and rapid—PP of CO2
in blood rises if respiration is not assisted. Ventilatory support with added
oxygen is frequently required. It tends to accentuate perfusionventilation mismatch
in the lungs by causing vasodilatation in hypoxic alveoli.
Pharyngeal and laryngeal reflexes are abolished early and
coughing is suppressed while bronchi dilate—preferred for asthmatics. It
inhibits intestinal and uterine contractions. This property is utilized for
assisting external or internal version during late pregnancy. However, its use
during labour can prolong delivery and increase postpartal blood loss.
Urine formation is
decreased during halothane anaesthesia—primarily due to low g.f.r. as a result
of fall in BP.
Hepatitis occurs in
susceptible individuals (approximately 1 in 10,000) especially after repeated
use. A metabolite of halothane is probably involved—causes chemical or
immunological injury.
A genetically
determined reaction malignant hyperthermia occurs rarely. Many susceptible subjects have an abnormal RyR
(Ryanodine receptor) calcium channel at the sarcoplasmic reticulum of the skeletal
muscles, which is triggered by halothane to release massive amounts of Ca2+
intracellularly causing persistent muscle contraction and increased heat
production. Succinylcholine accentuates the condition (see Ch. No. 25). Rapid external cooling, bicarbonate infusion, 100%
O2 inhalation and i.v. dantrolene are used to treat malignant hyperthermia.
About 20% of halothane
that enters blood is metabolized in the liver, the rest is exhaled out.
Elimination may continue for 24–48 hours after prolonged administration.
Recovery from halothane anaesthesia is smooth and reasonably quick; shivering
may occur but nausea and vomiting are rare. Psychomotor performance and mental
ability remain depressed for several hours after regaining consciousness.
It is currently one of
the most popular anaesthetics because of nonirritant, noninflammable, pleasant
and rapid action, particularly suitable for induction and maintenance in
children and as maintenance anaesthetic in adults. However, in affluent
countries it has been largely replaced by the newer agents which are costly.
Its deficiencies in terms of poor analgesia and muscle relaxation are
compensated by concomitant use of N2O or opioids and neuromuscular
blockers.
Enflurane
This faster acting
substitute of halothane has similar actions,
but is less soluble in blood and fat; accumulates in the body to a lesser extent.
Because of its propensity to provoke seizures at deeper levels of anaesthesia,
it has been superseded by isoflurane which has other desirable properties as
well.
Isoflurane (SOFANE)
It is a later
introduced (1981) isomer of enflurane; has similar properties, but about 1½
times more potent, more volatile and less soluble in blood. It produces
relatively rapid induction and recovery, and is administered through a special
vaporizer; 1.5–3% induces anaesthesia in 7–10 min, and 1–2% is used for maintenance.
Magnitude of fall in BP is similar to halothane, but is
primarily due to vasodilatation while cardiac output is well maintained. Heart
rate is increased. These cardiovascular effects probably result from
stimulation of β adrenergic receptors, but it does not sensitize the heart to
adrenergic arrhythmias. Coronary circulation is maintained: safer in patients
with myocardial ischaemia.
Respiratory depression is prominent and assistance is usually
needed to avoid hypercarbia. Secretions are slightly increased.
Uterine and skeletal muscle relaxation is similar to halothane.
Metabolism of isoflurane is negligible. Renal and hepatic
toxicity has not been encountered. Postanaesthetic nausea and vomiting is low.
Pupils do not dilate and light reflex is not lost even at deeper
levels.
Though slightly irritant, isoflurane has many advantages, i.e.
better adjustment of depth of anaesthesia and low toxicity. It is a good
maintenance anaesthetic, but not preferred for induction. It does not provoke
seizures and is preferred for neurosurgery. Isoflurane has become the routine
anaesthetic, but use may be restricted due to cost.
Desflurane
It is a newer all fluorinated congener of isoflurane
which has gained popularity as an anaesthetic for out patient surgery in
western countries. Though it is highly volatile, a thermostatically heated
special vaporizer is used to deliver a precise concentration of pure desflurane
vapour in the carrier gas (N2O + O2) mixture. Its
distinctive properties are lower oil: gas partition coefficient and very low
solubility in blood as well as in tissues, because of which induction and
recovery are very fast. Depth of anaesthesia changes rapidly with change in
inhaled concentration. Post-anaesthetic cognitive and motor impairment is
shortlived— patient can be discharged a few hours after surgery.
Desflurane is less potent than isoflurane; higher concentration
has to be used for induction—irritates air passage—may induce coughing, breath-holding
and laryngospasm because of somewhat pungent odour making it unsuitable for
induction. Rapid induction sometimes causes brief sympathetic stimulation and tachycardia.
Degree of respiratory depression, muscle relaxation, vasodilatation and fall in
BP, as well as maintained cardiac contractility and coronary circulation are
like isoflurane. Lack of seizure provoking potential or arrhythmogenicity and
absence of liver as well as kidney toxicity are also similar to isoflurane. It
is exhaled unchanged, but more rapidly. As such, desflurane can serve as a good
alternative to isoflurane for routine surgery as well, especially prolonged
operations.
Sevoflurane
This new polyfluorinated anaesthetic has properties
intermediate between isoflurane and desflurane. Solubility in blood and tissues
as well as potency are less than isoflurane but more than desflurane.
Induction and
emergence from anaesthesia are fast and rapid changes in depth can be achieved.
Absence of pungency makes it pleasant and administrable through face mask. Unlike
desflurane, it poses no problem in induction; acceptability is good even by
pediatric patients. Recovery is smooth; orientation, cognitive and motor
functions are regained almost as quickly as with desflurane. Sevoflurane is
suitable both for outpatient as well as inpatient surgery, but its high cost
and need for highflow open system makes it very expensive to use. In India,
only highend hospitals are using it.
Sevoflurane does not cause sympathetic stimulation and airway
irritation even during rapid induction. Fall in BP is due to vasodilatation as
well as modest cardiac depression. Respiratory depression, absence of seizure
and arrhythmia precipitating propensity are similar to isoflurane. About 3% of
absorbed sevoflurane is metabolized, but the amount of fluoride liberated is
safe for kidney and liver. However, it is degraded by sodalime—not recommended
for use in closed circuit.
Intravenous Anaesthetics Inducing Agents
These are drugs which on i.v. injection produce loss of
consciousness in one armbrain circulation time (~11 sec); are generally used
for induction because of rapidity of onset of action. Anaesthesia is then
usually maintained by an inhalational agent. They also serve to reduce the
amount of maintenance anaesthetic. Supplemented with analgesics and muscle
relaxants, they can also be used as the sole anaesthetic.
Thiopentone sod.
It is an ultrashort acting thiobarbiturate, highly
soluble in water yielding a very alkaline solution, which must be prepared
freshly before injection. Extravasation of the solution or inadvertent
intraarterial injection produces intense pain—necrosis and gangrene may occur.
Injected i.v. (3–5
mg/kg) as a 2.5% solution, it produces unconsciousness in 15–20 sec. Its
undissociated form has high lipid solubility— enters brain almost
instantaneously. Initial distribution depends on organ blood flow— brain gets
large amounts. However, as other less vascular tissues (muscle, fat) gradually
take up the drug, blood concentration falls and it back diffuses from the brain:
consciousness is regained in 6–10 min (t½ of distribution phase is 3 min).
On repeated injection,
the extracerebral sites are gradually filled up—lower doses produce anaesthesia
which lasts longer. Its ultimate disposal occurs mainly by hepatic metabolism
(elimination t½ is 7–12 hr), but this is irrelevant for termination of action
of a single dose. Residual CNS depression may persist for > 12 hr. The
patient should not be allowed to leave the hospital without an attendant before
this time.
Thiopentone is a poor
analgesic. Painful procedures should not be carried out under its influence
unless an opioid or N2O has been given; otherwise, the patient may
struggle, shout and show reflex changes in BP and respiration.
It is a weak muscle
relaxant; does not irritate air passages. Respiratory depression with inducing
doses of thiopentone is generally transient, but with large doses it can be
severe. BP falls immediately after injection mainly due to vasodilatation, but
recovers rapidly. Cardiovascular collapse may occur if hypovolemia, shock or
sepsis are present. It does not sensitize the heart to Adr, arrhythmias are
rare.
Thiopentone is a
commonly used inducing agent. It can be employed as the sole anaesthetic for
short operations that are not painful.
Adverse Effects
Laryngospasm occurs generally when respiratory secretions or
other irritants are present, or when intubation is attempted while anaesthesia
is light. It can be prevented by atropine premedication and administration of
succinylcholine immediately after thiopentone. Succinylcholine and thiopentone
react chemically—should not be mixed in the same syringe.
Shivering and delirium may occur during recovery. Pain in the postoperative
period is likely to induce restlessness; adequate analgesia should be provided.
Postanaesthetic nausea and vomiting are uncommon.
It can precipitate acute intermittent porphyria in susceptible
individuals—contraindicated.
Other Uses
Occasionally used for
rapid control of convulsions.
Gradual i.v. infusion of subanaesthetic doses can be used to
facilitate verbal communication with psychiatric patients and for
‘narcoanalysis’ of criminals; acts by knocking off guarding.
PENTOTHAL, INTRAVAL SODIUM 0.5, 1 g powder for making fresh
injectable solution.
Methohexitone
sod.
It is similar to
thiopentone, 3 times more potent, has a quicker and briefer
(5–8 min) action. Excitement during induction and recovery is more common. It
is more rapidly metabolized (t½ 4 hr) than thiopentone: patient may be
roadworthy more quickly.
Propofol
Currently, propofol
has super-seded thiopentone as
an i.v. anaesthetic, both for induction as well as maintenance. It is an oily
liquid employed as a 1% emulsion. Unconsciousness after propofol injection
occurs in 15–45 sec and lasts 5–10 min. Propofol distributes rapidly
(distribution t½ 2–4 min). Elimination t½ (100 min) is much shorter than that
of thiopentone due to rapid metabolism.
Intermittent injection or continuous infusion of propofol is frequently
used for total i.v. anaesthesia when supplemented by fentanyl. It lacks airway
irritancy and is particularly suited for outpatient surgery, because residual
impairment is less marked and shorter lasting. Incidence of postoperative
nausea and vomiting is low; patient acceptability is very good. Excitatory effects
and involuntary movements are noted in few patients. Induction apnoea lasting
~1 min is common. Fall in BP due primarily to vasodilatation with less marked cardiac
depression occurs consistently, and is occasionally severe, but short lasting.
Bradycardia is also frequent. Maintenance anaesthesia with propofol produces
dose-dependent respiratory depression which is more marked than with thiopentone.
Pain during injection is also frequent; can be minimized by combining with lidocaine.
Dose: 2 mg/kg bolus i.v. for
induction; 9 mg/kg/hr for maintenance.
PROPOVAN 10 mg/ml and
20 mg/ml in 10, 20 ml vials.
In subanaesthetic doses (2.4 mg/kg/hr) it is the drug of choice
for sedating intubated patients in intensive care units. However, it is not
approved for such use in children; prolonged sedation with higher doses has
caused severe metabolic effects and heart failure even in adults.
Etomidate
It is another induction anaesthetic, which has a briefer
duration of action (4–8 min) than thiopentone; produces little cardiovascular
and respiratory depression, but motor restlessness and rigidity is more
prominent as are pain on injection or nausea and vomiting on recovery. It is a
poor analgesic and has not found much favour.
Slower Acting Drugs
Benzodiazepines (BZDs)
In addition to preanaesthetic
medication, BZDs are now frequently used for inducing, maintaining and
supplementing anaesthesia as well as for ‘conscious sedation’. Relatively large
doses (diazepam 0.2–0.3 mg/kg or equivalent) injected i.v. produce sedation,
amnesia and then unconsciousness in 5–10 min. If no other anaesthetic or opioid
is given, the patient becomes responsive in 1 hr or so due to redistribution of
the drug (distribution t½ of diazepam is 15 min), but amnesia persists for 2–3
hr and sedation for 6 hr or more. Recovery is further delayed if
larger doses are given. BZDs are poor analgesics : an opioid or N2O
is usually added if the procedure is painful.
By themselves, BZDs donot markedly depress respiration, cardiac
contractility or BP, but when opioids are also given these functions are considerably
compromised. BZDs decrease muscle tone by central action, but require neuromuscular
blocking drugs for muscle relaxation of surgical grade. They do not provoke
postoperative nausea or vomiting. Involuntary movements are not stimulated.
BZDs are now the preferred drugs for endoscopies, cardiac
catheterization, angiographies, conscious sedation during local/regional anaesthesia,
fracture setting, ECT, etc. They are a frequent component of balanced
anaesthesia employing several drugs. The anaesthetic action of BZDs can be
rapidly reversed by flumazenil 0.5–2 mg i.v.
Diazepam 0.2–0.5 mg/kg by slow
undiluted injection in a running
i.v. drip: this technique reduces the burning sensation in the vein and
incidence of thrombophlebitis.
VALIUM, CALMPOSE 10
mg/2 ml inj.
Lorazepam Three times more potent, slower acting and less irritating than diazepam. It
distributes more gradually—awakening may be delayed. Amnesia is more profound.
Dose 2–4 mg (0.04 mg/kg)
i.v. CALMESE 4 mg/2 ml inj.
Midazolam This BZD is water soluble, nonirritating to
veins, faster and shorter acting and 3 times more potent than diazepam. It is
being preferred over diazepam for anaesthetic use: 1–2.5 mg i.v. followed by
1/4th supplemental doses. Also used for sedation of intubated and mechanically
ventilated patients and in other critical care anaesthesia as 0.02–0.1 mg/kg/hr
continuous i.v. infusion.
FULSED, MEZOLAM, SHORTAL 1 mg/ml, 5 mg/ml inj.
Ketamine
It is
pharmacologically related to the hallucinogen
phencyclidine; induces a so called ‘dissociative
anaesthesia’ characterized by profound analgesia, immobility, amnesia with
light sleep and feeling of dissociation from ones own body and the surroundings.
The primary site of action is in the cortex and subcortical areas; not in the
reticular activating system (site of action of barbiturates).
Respiration is not depressed, airway reflexes are maintained,
muscle tone increases; limb movements occur and eyes may remain open.
Heart rate, cardiac
output and BP are elevated due to sympathetic stimulation. A dose of 1–3
(average 1.5) mg/kg i.v. or 5 mg/kg i.m. produces the above effects within a minute,
and recovery starts after 10–15 min, but patient remains amnesic for 1–2 hr.
Emergence delirium, hallucinations and involuntary movements occur in upto 50%
patients during recovery; but the injection is not painful. Children tolerate
the drug better. Ketamine is metabolized in the liver and has an elimination t½
of 3–4 hr.
Ketamine has been used
for operations on the head and neck, in patients who have bled, in asthmatics
(relieves bronchospasm), in those who do not want to lose consciousness and for
short operations. It is good for repeated use; particularly suitable for burn
dressing. Combined with diazepam, it has found use in angiographies, cardiac
catheterization and trauma surgery. It may be dangerous for hypertensives, in
ischaemic heart disease and in those with raised intracranial pressure (it
increases cerebral blood flow), but is good for hypovolemic patients.
KETMIN, KETAMAX,
ANEKET 50 mg/ml in 2 ml amp, 10 ml vial.
Fentanyl
This short acting
(30–50 min) potent opioid analgesic
related to pethidine is generally given i.v. at the beginning of painful
surgical procedures. Reflex effects of painful stimuli are abolished. It is
frequently used to supplement anaesthetics in balanced anaesthesia. This
permits use of lower anaesthetic concentrations with better haemodynamic
stability. Combined with BZDs, it can obviate the need for inhaled anaesthetics
for diagnostic, endoscopic, angiographic and other minor procedures in poor
risk patients, as well as for burn dressing. Anaesthetic awareness with
dreadful recall is a risk.
After i.v. fentanyl (2–4 μg/kg) the patient
remains drowsy but conscious and his cooperation can be commanded. Respiratory
depression is marked, but predictable; the patient may be encouraged to breathe
and assistance may be provided. Tone of chest muscles may increase with rapid fentanyl
injection: a muscle relaxant is then required to facilitate mechanical
ventilation. Heart rate decreases, because fentanyl stimulates vagus. Fall in
BP is slight and heart is not sensitized to Adr. Supplemental doses of fentanyl
are needed every 30 min or so, but recovery is prolonged after repeated doses.
Nausea, vomiting and
itching often occurs during recovery. The opioid antagonist naloxone can be
used to counteract persisting respiratory depression and mental clouding.
Fentanyl is also employed as adjunct to spinal and nerve block anaesthesia, and
to relieve postoperative pain.
TROFENTYL, FENT 50 μg/ml in 2 ml amp, 10
ml vial. In the past fentanyl was combined with the short acting
neuroleptic droperidol to produce neurolept
analgesia. Since the combination produces marked fall in BP, respiratory depression and occasionally
cardiac arrhythmia, it is outmoded.
Alfentanil, Sufentanil and remifentanil are
still shorter acting analogues which
can be used in place of fentanyl.
Dexmedetomidine
Activation of central α2 adrenergic receptors
has been known to cause sedation and analgesia. Clonidine (a selective α2 agonist antihypertensive)
given before surgery reduces anaesthetic requirement. Dexmedetomidine is a
centrally active selective α2A agonist that has been
recently introduced for sedating critically ill/ventilated patients in
intensive care units. Analgesia and sedation are produced with little
respiratory depression, amnesia or anaesthesia. It is administered by i.v.
infusion. Side effects are similar to those with clonidine, viz. hypotension, bradycardia and dry
mouth.
Conscious Sedation
‘Conscious sedation’ is a monitored state of altered consciousness
that can be employed (supplemented with local/regional anaesthesia), to
carryout diagnostic/short therapeutic/dental procedures in apprehensive
subjects or medically compromised patients, in place of general anaesthesia. It
allows the operative procedure to be performed with minimal physiologic and
psychologic stress. In conscious sedation, drugs are used to produce a state of
CNS depression (but not unconsciousness), sufficient to withstand the trespass
of the procedure, while maintaining communication with the patient, who at the
same time responds to commands and is able to maintain a patent airway. The difference
between conscious sedation and anaesthesia is one of degree. The protective
airway and other reflexes are not lost, making it safer. Drugs used for
conscious sedation are:
Diazepam
It is injected i.v. in small (1–2 mg) repeated doses or by slow infusion until the desired
level of sedation is produced indicated by relaxation, indifference, slurring
of speech, ptosis, etc. Further injection is stopped, after which this state
lasts for about 1 hour and psychomotor impairment persists for 6–24 hours; an
escort is needed to take back the patient home. Flumazenil can be used to
reverse the sedation, but repeated doses are needed.
Midazolam (i.v.) is a shorter acting alternative to diazepam.
Oral diazepam administered 1 hr before is also used with the limitation that
level of sedation cannot be titrated. The patient remains sedated (not
roadworthy) for several hours.
Propofol
Because of brief action, it has to be
administered by continuous i.v. infusion regulated by infusion
pump throughout the procedure. However, level of sedation can be altered during
the procedure and recovery is relatively quick, permitting early discharge of
the patient.
Nitrous oxide
The patient is made to breathe 100% oxygen through a nose
piece or hood and N2O is added in 10% increments (to a maximum of
50%) till the desired level of sedation assessed by constant verbal contact is
obtained. This is maintained till the procedure is performed. Thereafter, N2O
is switched off, but 100% O2 is continued for next 5 min. The patient
is generally roadworthy in 30–60 min.
Fentanyl
Injected i.v. (1–2 μg/kg every 15–30 min), it can be used alone
or in combination with midazolam/ propofol.
Complications Of General Anaesthesia
A) During Anaesthesia
1.
Respiratory depression and hypercarbia.
2.
Salivation, respiratory secretions—less now as
nonirritant anaesthetics are mostly used.
3.
Cardiac arrhythmias, asystole.
4.
Fall in BP
5.
Aspiration of gastric contents: acid pneumonitis.
6.
Laryngospasm and asphyxia.
7.
Awareness: dreadful perception and recall of
events during surgery—by use of light anaesthesia + analgesics and muscle
relaxants.
8. Delirium,
convulsions and other excitatory effects are generally seen with i.v. anaesthetics—especially
if phenothiazines or hyoscine have been given in premedication.These are
suppressed by opioids.
9. Fire and
explosion—rare now due to use of noninflammable agents.
B) After Anaesthesia
1. Nausea and vomiting.
2. Persisting sedation:
impaired psychomotor function.
3. Pneumonia,
atelectasis.
4. Organ toxicities:
liver, kidney damage.
5. Nerve palsies—due to
faulty positioning.
6. Emergence delirium.
7. Cognitive defects: prolonged
excess cognitive decline has been observed in some patients, especially the
elderly, who have undergone general anaesthesia, particularly of long duration.
Drug Interactions
1. Patients on
antihypertensives given general anaesthetics—BP may fall markedly.
2. Neuroleptics, opioids,
clonidine and monoamine oxidase inhibitors potentiate anaesthetics.
3. Halothane sensitizes
heart to Adr.
4. If a patient on corticosteroids
is to be anaesthetized, give 100 mg hydrocortisone intraoperatively because
anaesthesia is a stress—can precipitate adrenal insufficiency and
cardiovascular collapse.
5. Insulin need of a
diabetic is increased during GA: switch over to plain insulin even if the patient
is on oral hypoglycaemics.
Preanaesthetic Medication
Preanaesthetic
medication refers to the use of drugs before anaesthesia to make it more
pleasant and safe. The aims are:
1.
Relief of anxiety and apprehension preoperatively
and to facilitate smooth induction.
2.
Amnesia for pre and postoperative events.
3.
Supplement analgesic action of anaesthetics
and potentiate them so that less anaesthetic is needed.
4.
Decrease secretions and vagal stimulation
caused by anaesthetics.
5.
Antiemetic effect extending to the postoperative
period.
6.
Decrease acidity and volume of gastric juice
so that it is less damaging if aspirated.
Different drugs
achieve different purposes. One or more drugs may be used in a patient depending
on the needs.
1. Sedative-antianxiety
Drugs
Benzodiazepines like
diazepam (5–10 mg oral) or lorazepam (2 mg or 0.05 mg/kg i.m. 1 hour before)
have become popular drugs for preanaesthetic medication because they produce
tranquility and smoothen induction; there is loss of recall of perioperative events
(especially with lorazepam) with little respiratory depression or accentuation
of postoperative vomiting. They counteract CNS toxicity of local anaesthetics
and are being used along with pethidine/fentanyl for a variety of minor surgical
and endoscopic procedures.
Midazolam is a good
amnesic with potent and shorter lasting action; it is also better suited for
i.v. injection, due to water solubility.
Promethazine (50 mg i.m.) is an
antihistaminic with sedative,
antiemetic and anticholinergic properties. It causes little respiratory
depression.
2. Opioids
Morphine (10 mg) or
pethidine (50–100 mg), i.m. allay
anxiety and apprehension of the operation, produce pre and postoperative
analgesia, smoothen induction, reduce the dose of anaesthetic required and supplement
poor analgesic (thiopentone, halothane) or weak anaesthetics (N2O).
Postoperative restlessness is also reduced.
Disadvantages They depress
respiration, interfere with pupillary signs of
anaesthesia, may cause fall in BP during anaesthesia, can precipitate asthma
and tend to delay recovery. Other disadvantages are lack of amnesia, flushing,
delayed gastric emptying and biliary spasm. Some patients experience dysphoria.
Morphine particularly contributes to postoperative constipation, vomiting and urinary
retention. Tachycardia sometimes occurs when pethidine has been used.
Use of opioids is now
mostly restricted to those having preoperative pain. When indicated, fentanyl
is mostly injected i.v. just before induction.
3. Anticholinergics
Atropine or hyoscine
(0.6 mg i.m./i.v.) have
been used, primarily to reduce salivary and bronchial secretions. Need for
their use is now less compelling because of the increasing employment of nonirritant
anaesthetics. However, they must be given before hand when ether is used. The
main aim of their use now is to prevent vagal bradycardia and hypotension
(which occur reflexly due to certain surgical procedures), and prophylaxis of
laryngospasm which is precipitated by respiratory secretions. Hyoscine, in
addition, produces amnesia and antiemetic effect, but tends to delay recovery.
Some patients get disoriented; emergence delirium is more common. They dilate
pupils, abolish the pupillary signs and increase chances of gastric reflux by
decreasing tone of lower esophageal sphincter (LES). They should not be used in
febrile patients. Dryness of mouth in the pre and postoperative period may be
distressing.
Glycopyrrolate (0.1–0.3 mg i.m.) is a
longer acting quaternary atropine
substitute. It is a potent antisecretory and antibradycardiac drug; acts
rapidly and is less likely to produce central effects (see Ch. No. 8).
4. Neuroleptics
Chlorpromazine (25 mg), triflupromazine (10 mg) or haloperidol
(2–4 mg) i.m. are infrequently used in premedication. They allay anxiety, smoothen
induction and have antiemetic action. However, they potentiate respiratory
depression and hypotension caused by the anaesthetics and delay recovery.
Involuntary movements
and muscle dystonias can occur, especially in children.
5. H2
Blockers
Patients undergoing prolonged operations, caesarian section and obese
patients are at increased risk of gastric regurgitation and aspiration
pneumonia. Ranitidine (150 mg) or famotidine (20 mg) given night before and in
the morning benefit by raising pH of gastric juice; may also reduce its volume
and thus chances of regurgitation. Prevention of stress ulcers is another
advantage. They are now routinely used before prolonged surgery.
The proton pump
inhibitor omeprazole/ pantoprazole is an alternative.
6. Antiemetics
Metoclopramide 10–20 mg i.m. preoperatively is effective in reducing postoperative
vomiting. By enhancing gastric emptying and tone of LES, it reduces the chances
of reflux and its aspiration. Extrapyramidal effects and motor restlessness can
occur. Combined use of metoclopramide and H2 blockers is more
effective.
Domperidone
is nearly as effective and does not produce extrapyramidal side effects.
After its success in
cancer chemotherapy induced vomiting, the selective 5HT3 blocker Ondansetron (4–8 mg i.v.) has been found
highly effective in reducing the
incidence of post anaesthetic nausea and vomiting as well (see Ch. No. 47).
Related Topics
