Identification of Events
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: PEM in New Zealand
The IMMP methodology is unique in that events in patients taking the monitored medicines are identified from several different sources.
IDENTIFICATION OF EVENTS
DEFINITION
The
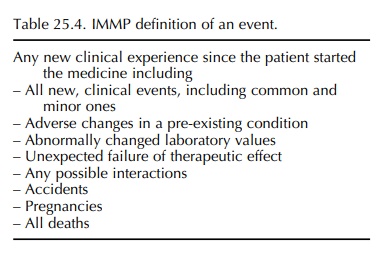
IMMP definition of an event is similar to that used in PEM in the UK (Chapter
24) – any new clin-ical experience since the patient started the medicine,
whether the event is thought to be drug related. This definition incorporates
several possible clinical outcomes which are summarized in Table 25.4.
INTENSIVE METHODOLOGY FOR IDENTIFYING EVENTS
The IMMP methodology is unique in that events in patients taking the monitored medicines are identified from several different sources. The primary method is by follow-up questionnaires to patients’ doctors (as in UK PEM), but the IMMP also identifies adverse events from spontaneous reporting, from duplicate prescriptions, from other pharmacy data and also from data linkage to national morbidity and mortality databases. This intensive methodology for identifying adverse events is described in this section.
FOLLOW-UP QUESTIONNAIRES
Questionnaires
seeking information on adverse events are sent to patients’ doctors at regular
intervals. Most often this is the patient’s general practitioner (GP) but may
be another prescribing doctor, includ-ing specialists. Doctors are asked to
record all new clinical events (Table 25.4) in the patient’s notes from a
specified date. For a new patient, this will usually be from the commencement
of therapy, but if questionnaires have been sent previously, doctors are
requested to record events from the date of the last received questionnaire
(this date is given to facilitate record searching). For drugs used
inter-mittently or if there is no follow-up information in the notes, the
doctor (or practice nurse) may contact the patient directly to obtain the
information required.
The compliance rate for returning IMMP ques-tionnaires has always been very high (greater than 80% for many medicines) and is currently around 70%. This average response rate is higher than that normally obtained in the UK PEM programme and may be related to several factors, including the high spontaneous reporting rate observed in the NZ (Olsson, 1999). Doctors are not paid for complet-ing the questionnaires, but they are now able to claim Continuing Medical Education (CME) points for completed forms.
SPECIFIC QUESTIONNAIRES
Whilst
all IMMP follow-up questionnaires have simi-lar core elements (e.g. patient,
prescription and doctor details) and are designed primarily to obtain
infor-mation on adverse events, each questionnaire for a specific medicine is
designed according to other outcomes of interest. Thus, questionnaires for
intra-uterine devices (IUDs) included questions about preg-nancies and for
sibutramine included questions on body mass index (BMI). Some questionnaires
(e.g. for antipsychotic medicines) have sought information on indication for
use of the medicine and concurrent medications. All questionnaires request
information on the cessation of therapy and reasons for stopping treatment.
SUPPLEMENTARY QUESTIONNAIRES
It
is sometimes necessary to obtain additional informa-tion to that obtained from
the standard questionnaires described above. Examples include
• Baseline and serial liver function tests for tolcapone.
• Asthma severity questionnaire for salmeterol and eformoterol.
• Results of endoscopy checks for omeprazole.
• Questions about previous history of peptic ulcer disease for the COX II inhibitors.
If
pregnancies are reported on the standard ques-tionnaires (questions are
incorporated for women of child-bearing age), then further information is
sought regarding the outcome of the pregnancy. For reported deaths, further
information on the cause of death and possible relationship with the medicine
is sought by further questionnaires and also by linkage to mortality databases
(see DATA LINKAGE).
DUPLICATE PRESCRIPTIONS
During
the 1980s, the IMMP developed duplicate prescription pads to enhance event
reporting. In partic-ular, regions of NZ (covering about 25% of the popu-lation)
personalized prescription pads were given to GPs, private specialists and
hospitals (printed with the name of the hospital). Doctors gave the origi-nal
and copy of the prescription to the patient who took both to the pharmacist,
and then the accumu-lated copies were sent to the IMMP. An early study showed
that the event-reporting rate in the duplicate prescription region was 14 times
greater than that in a non-duplicate region (Coulter, 1986). This is because on
duplicate prescription forms prescribers are asked to record any adverse events
at the time of the consultation (Coulter, 1998) thus increasing the number of
event reports received by the IMMP. The IMMP prescription pads have now become
supplanted by electronic prescribing. Computer software for prac-titioners
flags the monitored medicine and prints out a duplicate prescription whenever a
monitored medicine is prescribed.
INTENSIFIED SPONTANEOUS REPORTING
Spontaneous
reports sent to the NZ Pharmacovigi-lance Centre currently comprise about 12%
of the total reports received for IMMP medicines. These ‘yellow card’ reports
may come from health professionals, patients/carers or pharmaceutical
companies. They are often of great value as they highlight a specific clini-cal
concern and may form the index case for a series being considered as a signal.
Doctors
and other health professionals in NZ are made aware which medicines are being
monitored by the IMMP via listings in the Ministry of Health publication Prescriber Update and in the MIMS Cata-logue (the latter is linked to
most patient management systems). In addition, drug companies are required to
state that their medicine is being monitored by IMMP in all company product
information, and visiting company representative are asked to remind doctors.
The inclusion of a medicine in the programme should thus increase the
spontaneous reporting rate, and an early study of betaβ-blockers showed this to
be the case (Coulter and McQueen, 1982).
The
symbiotic relationship between the IMMP and the NZ spontaneous reporting
programme (CARM) is much valued as both programmes benefit from work-ing
together in the same national pharmacovigilance unit. This is a key difference
to the UK PEM scheme, which operates entirely separately to the UK sponta-neous
reporting scheme.
DATA LINKAGE
In
NZ, there are national databases containing infor-mation on births, deaths,
hospital admissions and other morbidity outcomes, e.g. cancer. Identification
of patients in these databases is by their unique NHI number, which – as
discussed earlier – is now avail-able for the vast majority of patients. It is
therefore possible to obtain mortality and morbidity data for patients in the
IMMP cohorts by record linkage to these databases. This has proved very useful
for iden-tifying which patients have died whilst taking a moni-tored medicine,
and additional information is routinely sought on the cause of death. In
addition to enabling the identification of events that may not be picked up
from other sources, data linkage also offers great opportunity for other
pharmacoepidemiology stud-ies of patients in the IMMP cohorts (see SPECIFIC STUDIES USING INTENSIVE MEDICINES MONI-TORING PROGRAMME DATA).
Related Topics
