Routine Analysis of Data
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: PEM in New Zealand
At various time points in each monitoring study, the IMMP performs routine analyses of both the expo-sure (prescription) and the outcome (events) data.
ROUTINE ANALYSIS OF DATA
At
various time points in each monitoring study, the IMMP performs routine
analyses of both the expo-sure (prescription) and the outcome (events) data.
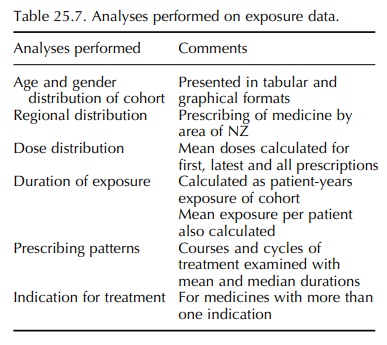
The analyses performed on the exposure data are summarized in Table 25.7.
ANALYSES OF OUTCOME DATA
The main outcome analysed is adverse events whilst the patient is taking the medicine (and for a feasible period after stopping). There are however additional endpoints analysed – e.g., reasons for the cessation of therapy and failure of therapeutic response.
·
Table of all events: A listing of all
the individ-ual events is presented by system/organ class, and within the
classes the events are sorted into clin-ically related groupings. This allows a
clinically orientated visual assessment of the events reported and is useful in
signal detection. It shows, for every event, the age and gender of the patient,
the dose, the duration to onset and the relationship that was established at
the time of the review of the report. It also shows deaths and withdrawals. The
individ-ual events can be cross referenced with the table of reports, which
presents the events in the context of the whole reaction.
·
Table of reports: This is a listing
by report and shows all the events
associated with each report, e.g. one report describing eosinophilia,
arthralgia, malaise and rash with omeprazole, thus presenting the events in the
context of the whole reaction. The age and gender for each patient is shown
along with the dose, severity, relationship and outcome of each event.
·
Profile of adverse
events:
This provides a table and histogram
showing numbers and rates by system/organ class of reactions, incidents and all
events, respectively.
·
Incidence of adverse
reactions:
This provides a listing of all events
assessed as reactions, showing the percentage of each within each system/organ
class, the percentage of each reaction amongst all reports and the rate of
occurrence of each reac-tion. These are sorted into clinical groupings within
each class.
·
Most frequent events: These are shown
(usually the top 10) with numbers and
rates together with the numbers and rates of withdrawals and deaths for these
events.
· Reporting rates: For each drug monitored, rates per 1000 patients are calculated for the numbers of (a) reports, (b) all events, (c) reactions and incidents, respectively, in total and by gender. The overall reaction and incident rates are useful for comparing subgroups and for between-drug comparisons, but in contrast to rates for individual events, do not provide a specific measure of risk because some patients have several events associ-ated with the one report.
THE IMPORTANCE OF REPORTING RATES
The
profiles of adverse events for a drug are differ-ent at high- and low-reporting
rates. At specific rates of reporting, some events are more likely to be
reported than others and, equally important, some are less likely to be
reported. The IMMP provides a unique opportunity for comparing the rates of
IMMP (intensified) spontaneous reporting of specific events with the rates from
using PEM question-naires. Angioedema/urticaria, extrapyramidal effects and
blood dyscrasias were as likely to be reported spontaneously as with PEM.
Conversely, cardiac dysrhythmias, dry mouth, dyspepsia, constipation, death and
events suggesting immunological disorders were, by comparison, very unlikely to
be reported spontaneously. Other events ranged between these two extremes. It
needs to be emphasized that this refers to IMMP ‘intensified’ spontaneous
reporting, which has a higher rate of reporting than the standard spontaneous
reporting programme in NZ. It follows therefore that studies on specific drugs
are not compa-rable unless the reporting rates are similar. Similarly, rates of
reporting may provide a guide as to what types of reactions may have been
missed.
Related Topics
