Outputs of PEM in New Zealand
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: PEM in New Zealand
There are several key elements to successful identification of previously unrecognized adverse reactions in the IMMP.
OUTPUTS OF PEM IN NEW ZEALAND
SIGNAL IDENTIFICATION
There
are several key elements to successful identi-fication of previously
unrecognized adverse reactions in the IMMP. These have been reviewed recently
(Clark and Harrison-Woolrych, 2006) and include (a) the intensive methodology
used to obtain events from multiple sources (see above), (b) the high quality
and completeness of reports received by the IMMP and (c) the evaluation of
every event report by at least one clinical assessor. The IMMP does not rely on
auto-mated processes for signal identification, preferring regular clinical
assessment of event listings for each medicine from early in the monitoring
study. In addi-tion, analyses of ‘incidents’ – as outlined above – contributes
to the process at a later stage of monitoring.
Possible
signals first identified by individual clin-ical assessment are further
investigated by obtain-ing additional evidence from other sources. These might
include the reporting doctor (or other reporter), other databases including the
WHO-UMC interna-tional spontaneous reporting database, pharmaceuti-cal
companies, medicine regulatory bodies and the published literature. Using these
methods, the IMMP has had some success in signal identification, as outlined in
this section.
SIGNALS REPORTED TO THE NEW ZEALAND MEDICINES ADVERSE REACTIONS COMMITTEE
Signals
generated in 11 drugs between 1985 and 1995 were searched from the agenda
material and minutes of the MARC meetings and from publications. For the
purposes of this evaluation, a signal was recorded as such if the MARC was
alerted before the date of the second non-IMMP publication. The date that the
MARC was alerted to each signal was recorded, and this date was compared with
the date of the first two publications (if any) found by Medline and AdisBase
searches of the international literature (all languages with an English
abstract). Medline was searched from 1985 and AdisBase from 1989. Case reports
and clinical trials were included in the searches. Adis-Base searches included
publications from regula-tory authorities internationally. Data sheets were not
searched. The dates of any IMMP publications were also recorded. Any
recommendations of the MARC because of considering the signals were noted.
Events that are expected because of known pharmacologi-cal action (e.g. tremor
with beta-agonists) were not recorded as signals.
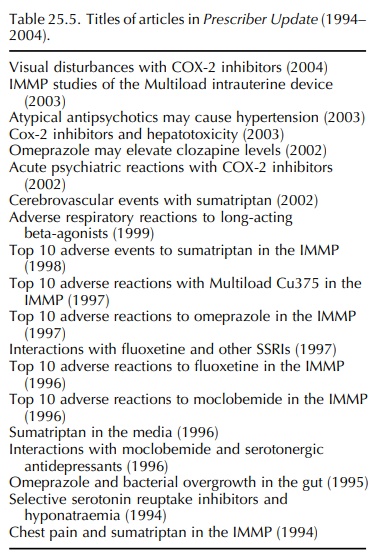
This
analysis identified 153 signals recorded in the 10-year period. Many of the
early signals were published in the NZ
Family Physician published by the Royal NZ College of General Practitioners
or in Prescriber Update (Table 25.5).
Of the 153 signals identified, 132
(86%) were notified to the MARC before any publication found in the
international liter-ature. Eighty-six (56%) of the signals have since been
strengthened or confirmed by at least one non-IMMP publication. In 72 (47%)
instances, the IMMP publi-cation was the first report of the signal identified,
and in 23 (15%) it was the second. On 39 (25%) occa-sions, the MARC recommended
action after consider-ing the signals. These included articles in Prescriber Update, writing to pharmaceutical companies for further information, changes to data sheets and further
investigations.
PUBLISHED SIGNALS BEFORE 1995
Early
signals published in the wider medical literature include cough and
angiotensin-converting enzyme (ACE) inhibitors (Coulter and Edwards, 1987), eye
pain with nifedipine (Coulter, 1988), ACE inhibitors and anaemia (Edwards and
Coulter, 1989), mianserin and agranulocytosis (Coulter and Edwards, 1990), the
intestinal effects of captopril (Edwards, Coulter and Macintosh, 1992),
psoriasis with ACE inhibitors (Coulter and Pillans, 1993) and fluoxetine and
hyponatraemia (Pillans and Coulter, 1994).
RECENTLY IDENTIFIED SIGNALS
Signals published in the international literature during the last 11 years (from 1995 to 2006) include hypertension with moclobemide (Coulter and Pillans, 1995b), fluoxetine and extrapyramidal effects (Coul-ter and Pillans, 1995a), acute psychiatric reactions with the COX II inhibitors (Coulter, 2002), acute visual impairment with rofecoxib and celecoxib (Coulter, Clark and Savage, 2003), psoriasis associ-ated with rofecoxib use (Clark and Coulter, 2003), the activation of pain by sumatriptan (Coulter et al., 2003), nose bleeds associated with risperi-done (Harrison-Woolrych and Clark, 2004), amnesia associated with sibutramine (Clark and Harrison-Woolrych, 2004), QT interval prolongation associated with sibutramine (Harrison-Woolrych et al., 2006) and cardiac dysrhythmias with COX II inhibitors (Savage, Coulter and Harrison-Woolrych, 2005).
VALIDATION OF SIGNALS
Investigating Signals by Survey of Cohort Sample
The
IMMP cohorts offer a great opportunity to further investigate signals
identified early in the monitoring process. Such studies aim to estimate
incidence or prevalence of specific adverse reactions and may also investigate
risk factors for these reactions. Follow-ing a cluster of reports of nocturnal
enuresis (bed wetting) associated with the atypical antipsychotic medicine
clozapine, the IMMP is now further inves-tigating this signal. Cohorts of
patients taking clozap-ine, olanzapine, quetiapine or risperidone during 2003
have been established, and follow-up questionnaires with additional specific
questions about bed wetting have been sent to patients’ doctors. It was
considered necessary to add specific questions for doctors/mental health nurses
to ask the patients directly, as enuresis is an embarrassing problem which is
unlikely to be spontaneously reported. This study will enable calcu-lation of
the prevalence of enuresis in patients taking clozapine (and identify risk
factors for this adverse event) and will allow comparison with three other
atypical antipsychotics.
Use of Prescription History
The
evaluation of 50 reports coded as ‘tolerance’ with sumatriptan was facilitated
by having a longitudinal record of prescription data with the numbers of
tablets or injections dispensed recorded for many patients over a period of
several years. The reports described patients who claimed that over a period of
months or years the drug did not work as well as it did initially and they
required higher or more doses to relieve an attack of migraine, or the drug did
not work at all. In the natural history of the disease, there are fluctuations
in frequency and severity of attacks, and so these reports were difficult to
interpret. It was felt that if there was any general trend to tolerance, then
mean usage per patient over time would increase.
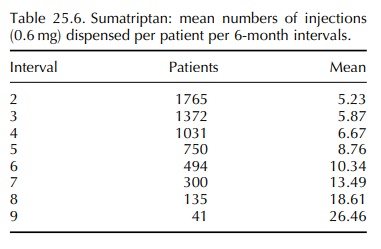
The
prescription data were therefore analysed, and the mean number of injections or
tablets (100 mg equivalent) per patient per 6-month interval was calcu-lated.
The results for those patients who had used injections only are shown in Table
25.6 over a period of eight intervals, and an increase was demonstrated at each
interval. The first interval was omitted because it would be a trial period of
use and for many patients may not be typical of later use. The latest interval
was also excluded because it may not have been complete. The slope of the
changes was statistically significant for both the injections and the tablets,
but the changes were more marked for the injections. There were no identifiable
confounders (Coulter, DM, presentation at the 18th Annual Meeting of National
Centres Partic-ipating in the WHO International Drug Monitoring Programme,
Portugal, 1996).
Related Topics
