Indole Alkaloids
| Home | | Pharmacognosy |Chapter: Pharmacognosy and Phytochemistry : Drugs Containing Alkaloids
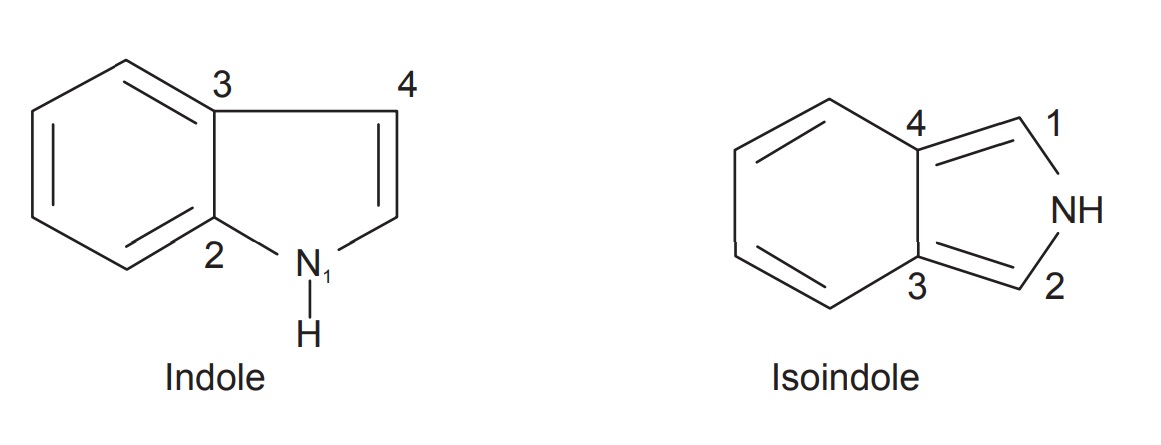
Indole (1-H-indole) is a benzopyrrole in which the benzene and pyrrole rings are through the 2, 3-positions of the pyrrole. The indole nucleus is found in a large number of naturally occurring compounds. It is of commercial importance as a component of perfumes. Isoindole (I-H-isoindole), the isomer in which the benzene and pyrrole rings are fused through the 3- and 4-positions of the pyrrole, is not stable. A few of its derivatives are known, the simplest being N-methylisoindole.
Indole Alkaloids
Indole (1-H-indole) is a benzopyrrole in which the benzene
and pyrrole rings are through the 2, 3-positions of the pyrrole. The indole
nucleus is found in a large number of naturally occurring compounds. It is of
commercial importance as a component of perfumes. Isoindole (I-H-isoindole),
the isomer in which the benzene and pyrrole rings are fused through the 3- and
4-positions of the pyrrole, is not stable. A few of its derivatives are known,
the simplest being N-methylisoindole.
Indole was first obtained (and its structure elucidated) in
1866 by Adolf von Baeyer. Interest in indole chemistry revived about 1930 when
it was discovered that the essential amino acid, tryptophan, the plant growth
hormone, heteroauxin, and several groups of important alkaloids are indole
derivatives. It was shown that 3-methylindole (skatole) is produced with indole
during pancreatic digestion or putrefactive decomposition of proteins and,
hence, both are found in the intestines and feces. Interest has centered on
medicinal and biochemical aspects of indole chemistry. Serotonin, which has
been identified as a metabolite in brain chemistry; the psychotomimetic
indoles, psilocin and psilocybin from mushrooms; the tranquilizer reserpine;
and the melanin pigments are a few of the compounds that have been studied.
Indole is a colourless crystalline solid (mp 52–54°C, bp
254°C). The heat of combustion at constant volume is 4,268 MJ/mol (10–20
kcal/mol). The molecule is planar and has only moderate polarity. Indole has
good solubility in a wide range of solvents including petroleum ether, benzene,
chloroform and hot water. The solubility in cold water is only 1:540 at 25°C;
thus, water is a good solvent for puri-fication by recrystallization. Indole
forms salts with high concentrations of both strong bases and strong acids.
The various plants containing indole alkaloids are vinca,
ergot, Rauwolfia, nux vomica, physostigma, etc.
