Iron
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Transition Metals and d-Block Meta Chemistry
Iron is the chemical element with the symbol Fe (Latin: ferrum) and atomic number 26. It is one of the most used metals because of the relatively low production costs and its high strength.
Iron
and ruthenium
Group 8 of the periodic table of elements consists of the
nonradioactive members iron (Fe), ruthenium (Ru) and osmium (Os), as well as the
radioactive element Hassium (Hs) (Figure 7.40).
All three nonradioactive elements are silvery white, hard
transition metals with a high melting point. Iron has been classified as the
most common element within the entire earth, as most of the earth’s core is
iron. In contrast, ruthenium and osmium are two of the rarest elements on
earth. The radioactive element hassium has not been isolated in pure form yet
and therefore its exact properties have not been established. It has only been
produced in nuclear reactors and never has been isolated.
The electronic configuration of group 8 metals is shown in Figure 7.41.
Iron
Iron is the chemical element with the symbol Fe (Latin: ferrum)
and atomic number 26. It is one of the most used metals because of the
relatively low production costs and its high strength. Iron can be found in
many everyday items, from food containers to screw drivers or any type of
machinery. Steel is a form of iron, which is alloyed with carbon and a variety of
other metals.
Iron ions are a necessary trace element used by almost all
living organisms with the only exceptions being a few prokaryotic organisms
that live in iron-poor conditions. As an example, the lactobacilli in iron-poor
milk use manganese for their catalysis processes. Iron-containing enzymes,
usually containing haeme prosthetic groups, participate in the catalysis of
oxidation reactions in biology and in the transport of a number of soluble
gases.
More than other metals, metallic iron has long been associated
with health. Chalybeate springs, the iron-containing waters, have been well
known for centuries for their healing properties. In the nineteenth century,
the ‘veritable pills of Blaud’, which contain ferrous sulfate and K2CO3,
were used to ‘cure everything’. In the 1930s, the relationship between
iron-deficiency anaemia and the lack of dietary iron was established. Nowadays,
iron deficiency is the most frequent nutritional deficiency in the world.
1. Chemistry of iron
Iron is a metal extracted from iron ore, and is almost never
found in the free elemental state. In order to obtain elemental iron, the
impurities must be removed by chemical reduction. Iron is the main component of
steel, and it is used in the production of alloys or solid solutions of various
metals.
Finely divided iron powder is pyrophoric in air. There is a
difference between when elemental iron is heated in dry air or in the presence
of humidity. Fe heated in dry air will oxidise, whilst when heated in moist air
it will rust characterised by the formation of Fe2O3 ⋅ xH2O.
The formation of rust is an electrochemical process occurring in the presence
of oxygen, water and an electrolyte such as NaCl.
2Fe → 2Fe2+ + 4e−
O2 + 2H2O + 4e− → 4[OH]−
Fe(OH)2 oxidizes to Fe2O3 ⋅ xH2O
The highest oxidation states of iron are Fe(VI) and Fe(IV),
whilst Fe(V) is very rare. Examples of com-pounds where Fe occupies this
oxidation state include [FeO4]2−, [FeO4]3−,
[FeO4]4− and [FeO3]2−. Oxidation
states +2 and +3 are the most commonly occurring oxidation states for Fe. The
old name for Fe(III) is ferric and for Fe(II) is ferrous – this is still
reflected in many drug names. Fe reacts with halogens under heat with the
formation of FeF3, FeCl3, FeBr3 and FeI2.
FeF3 is a white solid, whilst FeCl3 is a dark-green
hygroscopic solid. FeCl3 is an important precursor for any Fe(III)
chemistry.
Fe(II) halogens are typically synthesised by reacting Fe with
the relevant acid, HX, with the exception of FeI2, which can be
synthesised from the elements directly. FeF2 is a white solid,
sparingly soluble in water, whilst FeCl2 forms white, water-soluble,
hygroscopic crystals.
Fe + 2HX → FeX2 + H2
2. Iron performs many vital functions in the human body
Iron is an essential trace element for the human body. Haemoglobin is the oxygen-transport metalloprotein in the red blood cells; myoglobin facilitates the oxygen use and storage in the muscles; and cytochromes transport electrons. Iron is also an integral part of enzymes in various tissues. The average 70-kg adult body contains around 4200 mg of iron ions. The majority (65%) can be found as haemoglobin or myoglobin, which is classified as the functional iron .
Iron will pass the stomach and is absorbed predominantly in the
duodenum and upper jejunum. Beyond this point, intestinal bicarbonate elevates
the pH, rendering iron insoluble. Free iron is, as most metal ions, highly
toxic to the human body. Therefore, nature has created a sophisticated
transport and storage system which ensures that no free iron ions are present
in the blood stream. Iron ions absorbed from the GI tract are transported via
transferrins, which are Fe3+-containing metalloproteins, to the
storage vessels or until it is incorporated into haemoglobin. In the human
body, Fe3+ is stored mainly in the liver and spleen in form of
ferritin, which is a water-soluble metalloprotein and stores Fe3+.
Transferrins include the so-called serum
transferrins, for example, ovotransferrin (present in egg white) and
lactoferrin (present in milk), which can transport ∼40 mg of iron ions per day in humans. The
glycoprotein pro-tein is folded in such a way that there are two pockets
suitable for the coordination of Fe3+. Figure 7.42 shows how Fe3+
is coordinated within these pockets. Note a CO32− ligand
is essential for the binding mechanism.
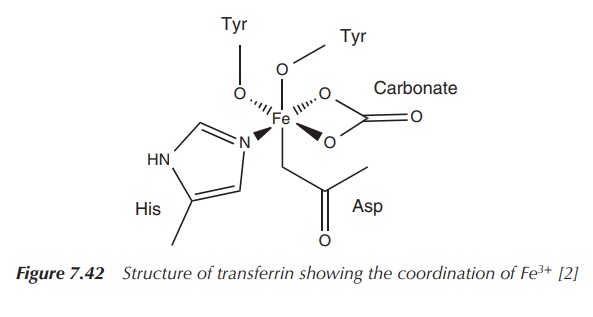
Transferrins are a group of proteins that are abundant in
blood and their primary role is to transport Fe3+, as free Fe3+ would be toxic to most organisms.
Transferrin consists of a single polypeptide chain inde-pendently binding a maximum
of two Fe3+
ions. Fe 3+ is
bound in a hexa-coordinated high-spin complex within the protein. The metal is
coordinated by the N atom from the imidazole residue, two deprotonated phenol
groups, a carboxylate and a carbonato ligand.
Haemoglobin and myoglobin are so-called haeme-iron proteins
characterised by the presence of a haeme group (a protoporphyrin group). The
main function of haemoglobin is the transport of oxygen to the tissue in need.
Myoglobin is the oxygen storage protein present in the tissue.
Myoglobin
consists of a monomeric protein chain containing one
protoporphyrin group as the functional unit.
Within myoglobin, the iron centre is coordinated by the four nitrogen groups of
the porphyrin in addition to the coordination of a fifth nitrogen centre from a
histidine (His) group. The functional unit containing the Fe(II) centre is
called a haeme group and is a
square-based pyramidal complex. During the oxygen binding mechanism, O2
will enter trans to the His group to give an octahedrally coordinated iron
species (Figure 7.43).
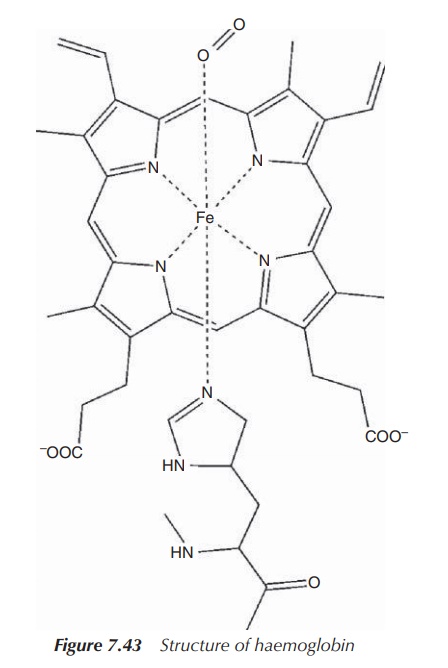
Haemoglobin, the oxygen-transport protein in the red blood cells, is a tetramer and each of the four chains contains a haeme group. It is interesting to note that the four haeme groups in haemoglobin do not operate independently. The release (and binding) of oxygen is a cooperative process, which means that the loss (uptake) of the first oxygen molecule triggers the release of the remaining three.
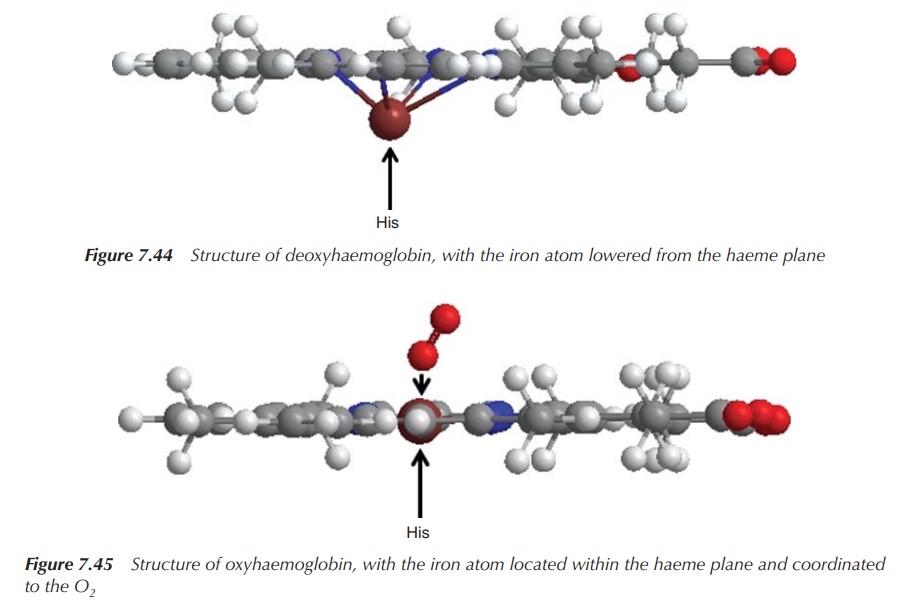
The current model for oxygen binding in haemoglobin and
myoglobin can be explained in the following way. The deoxy form contains a
high-spin Fe(II) centre, which, because of its size, does not form a plane with
its four nitrogen donor atoms. Instead, it is located slightly above the plane,
drawn towards the His residue. Once oxygen enters trans to the His residue, the
iron centre is oxidised to a low-spin Fe3+ centre and O2
is reduced to [O2]−. Both species contain an unpaired
electron. The low-spin Fe 3+ moves into the plane and pulls the His
residue down. This affects the remaining protein chain and triggers the
uptake/release of oxygen in the other three haeme groups (Figures 7.44 and 7.45).
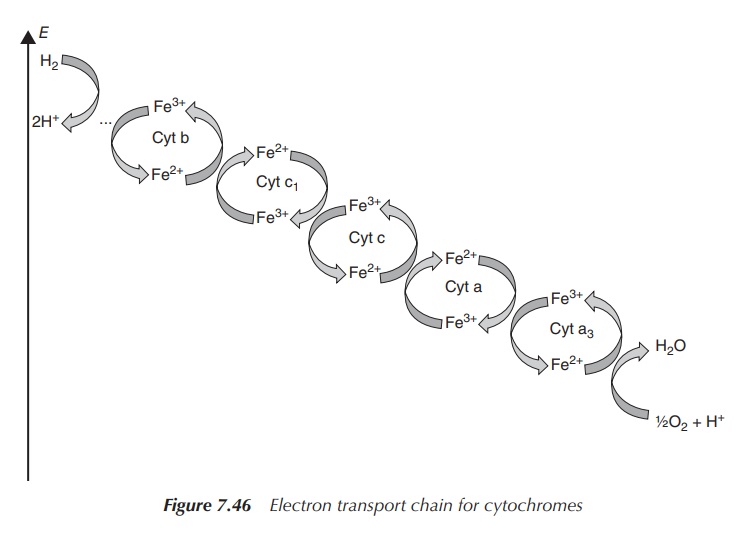
Cytochromes
are part of the mitochondrial electron-transfer chain but also
found in the chloroplasts of plants (involved
in photosynthesis). There are many different cytochromes; all are involved in
reduction–oxidation processes and are grouped into families – cytochromes a,
cytochromes b and cytochromes c. They contain a haeme group with an iron
centre, which has the ability to change reversibly from Fe(III) to Fe(II), and
vice versa. In contrast to the oxygen-coordinated iron centre in haemoglobin,
the iron in cytochromes is always six-coordinated. Cytochrome c, for example,
is involved in the mitochondrial electron-transfer chain and accepts an
electron from cytochrome c1 and transfers it to cytochrome c oxidase. This
electron is subsequently used in the reduction of oxygen, where four electrons
are needed. This means that actually four cytochrome c transfer an electron to
cytochrome c oxidase, where one molecule of O2 is converted to two
molecules of water. Note that Fe(III) forms the core of the oxidised
cytochromes whereas Fe(II) is present in the reduced form (Figure 7.46).
3. Iron uptake and metabolism
The normal diet contains around 15 mg of iron per day and only
around 10% is actually absorbed. The absorp-tion is dependent on a variety of
factors, such as its bioavailability, the amount of iron present in the food
and the body’s need for iron. There are phases within a human’s life where the
body requires an increased amount of iron, such as in pregnancy or growth
spurts. The absorption rate can be adjusted accordingly. The bioavail-ability
of iron is highly dependent on the food source. Meat, poultry and fish are rich
in easy-to-absorb iron. Absorption can also decrease depending on other dietary
components. Some compounds, such as polyphenols (in vegetables) or tannins (in
tea), can form chelates with iron.
Iron is actually excreted only in small amounts if there are no
major blood losses. The human body regulates the uptake of iron precisely, only
replacing the amount of iron lost in order to prevent an iron overload. Typical
examples for iron loss would be through superficial GI tract bleeding or
menstruation bleeding in women. Nevertheless, typically not more than 3 mg of
iron per day is lost.
4. Medicinal use
As discussed above, iron plays a vital role in the human body.
The lack of functional iron leads to anaemia, which is characterised by
lethargy and weakness. Usually, iron is administered orally as Fe2+
or Fe3+ salts. Fe2+ compounds are more soluble at
physiological pH. The advantage of Fe3+ salts is that they are not
prone to oxidation in aqueous solutions. The most common medicinal preparations
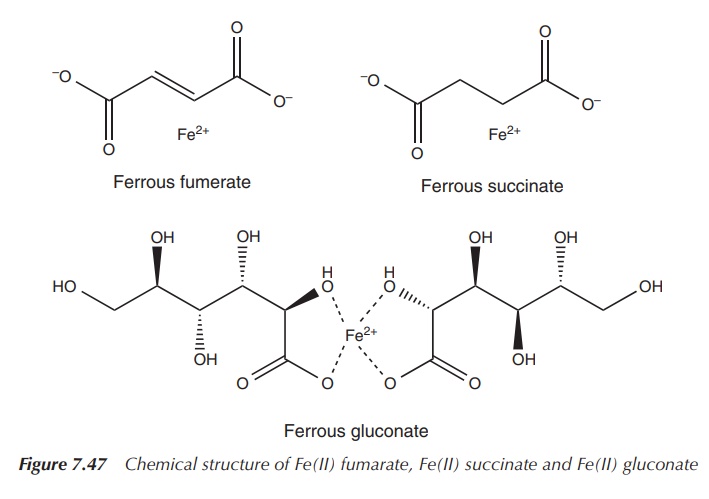
include FeCl3, FeSO4, Fe(II) fumarate, Fe(II) succinate
and Fe(II) gluconate (Figure 7.47).
The oral dose of Fe2+ for the treatment of iron-deficiency anaemia is typically recommended as 100–200 mg/day. In the case of ferrous sulfate (FeSO4), this is equal to 65 mg of Fe2+, which is given three times per day. As a therapeutic response, the haemoglobin concentration should raise about 100–200 mg/100 ml/day. It is recommended continuing the treatment for 3 months once the normal range is reached.
It is known that oral treatment with iron salts can
lead to GI irritations . There is very little difference between the efficiency
and absorbance rate of the above-mentioned iron salts. The choice of the preparation
is influenced by side effects and cost.
Iron salts such as iron dextran and iron sucrose can be
administered by IV infusion or IV injection. This administration route should
be chosen only when oral therapy is not successful, as there is a risk of
anaphy-lactic reactions. Patients with chronic renal failure who are on
haemodialysis treatment often require IV iron supplementation (Figure 7.48).
5. Bleomycin
The drug Bleomycin (BLM) is successfully used as an anticancer
agent, and is known to cause fragmentation of the DNA. The drug is used for the
treatment of testicular cancer, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and
cancers of the head and neck area (Cancer research UK). The name Bleomycin
describes a family of water-soluble antibiotics that can be isolated from the
bacterium Streptomyces verticillus.
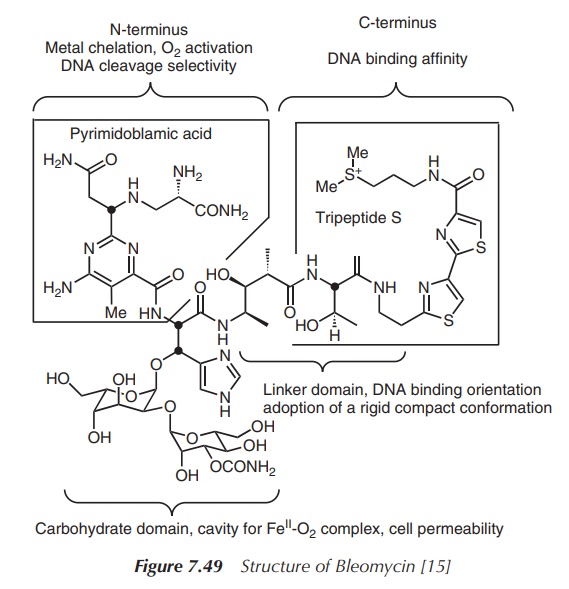
All family members contain the same core structure, a sulfur-containing
polypeptide chain, and are only differentiated by a small side group and the
sugar moiety (Figure 7.49).
BLM was discovered 1966 by Umezawa et al. when they screened the filtrate of S. verticillus for cytotoxic activity. The therapeutically active
forms of BLM are BLM A2 and B2, which differ only in the
side chain. BLM is believed to exhibit its anticancer activity by DNA
degradation, a process that is dependent on the presence of molecular oxygen,
and the binding of a metal to BLM to form the so-called ‘activated BLM complex’
.
The structure of BLM consists of several biologically important units, each contributing to its anticancer activity. Two structural units of importance to highlight are the metal-binding site and the DNA-binding site. It is believed that the intercalation of DNA by BLM occurs via the C-terminus, which contains two thiazole rings and the positively charged sulfonium salt. The positive charges of the sulfur atom can interact with the negatively charged phosphate backbones of the DNA. The metal-binding site can be found at the N-terminus and contains deprotonated amide and histidine groups. The metal is coordinated in a square planar complex, where a primary amine group occupies the axial position . It can coordinate to a variety of metals such as Cu2+, Co2+, Zn2+ and Fe2+, but it shows the highest binding affinity to Fe2+. The metal chelation and subsequent activation of molecular oxygen is crucial to the antiproliferative activity of BLM. The carbohydrate core seems to be less involved in the direct anticancer activity. Nevertheless, it has been suggested that it regulates the cellular uptake and indirectly regulates the anticancer activity.
With regard to the mode of action of BLM as an anticancer agent,
it is believed to be based on a unique radical mechanism that leads to DNA
fragmentation. In the presence of molecular oxygen, the ‘activated’ BLM
complex, HOO–Fe(III)BLM, is formed. This complex is known to cleave
double-stranded DNA at the 5′-GC or 5′-GT sites .
In the next step, the homolytic splitting of the O—O bond of the
HOO–Fe(III)BLM complex produces a radical that is able to degrade DNA. This
complex is known to cleave double-stranded DNA at the 5′-GC or 5′-GT
sites by abstraction of the C4′-H atom followed by a fragmentation
of the deoxyribose backbone . It is believed that the homolytic splitting of
the O—O bond and the cleavage of DNA take place in a concerted manner, which
means simultaneously. There are also other modes of action known for the DNA
degeneration by BLM, but the above-mentioned one is the most prominent one .
For a clinical application, usually a mixture
of BLM A2 (∼60–70%)
and BLM B2 (∼20–30%)
and others in combination with other antiproliferative agents are administered
J. C. Dabrowiak, Metals in medicine, Wiley-Blackwell, Oxford, 2009. The most
commonly used drug Blenoxane (70% BLM A2) has been developed and
marketed by GlaxoSmithKline and is used in the treatment of Hodgkin’s lymphoma,
testicular cancer and carcinomas of the skin and head and neck . BLM is usually
administered intravenously or intramuscularly. It is known to cause very little
bone marrow suppression, but has a high dermatological toxicity causing
increased pigmentation. The major side effect with the use of BLM is the
occurrence of pulmonary fibrosis, which is dose-related. It is therefore
important to regularly check the lungs by X-ray and respiratory function in
general .
Related Topics
