Regulation of Metabolism
| Home | | Biochemistry |Chapter: Biochemistry : Introduction to Metabolism and Glycolysis
The pathways of metabolism must be coordinated so that the production of energy or the synthesis of end products meets the needs of the cell.
REGULATION OF METABOLISM
The pathways of
metabolism must be coordinated so that the production of energy or the
synthesis of end products meets the needs of the cell. Furthermore, individual
cells do not function in isolation but, rather, are part of a community of
interacting tissues. Thus, a sophisticated communication system has evolved to
coordinate the functions of the body. Regulatory signals that inform an
individual cell of the metabolic state of the body as a whole include hormones,
neurotransmitters, and the availability of nutrients. These, in turn, influence
signals generated within the cell (Figure 8.5).
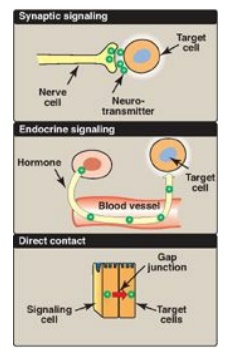
Figure 8.5 Some commonly used mechanisms for transmission of regulatory signals between cells.
A. Intracellular communication
The rate of a metabolic
pathway can respond to regulatory signals that arise from within the cell. For
example, the rate of a pathway may be influenced by the availability of
substrates, product inhibition, or alterations in the levels of allosteric
activators or inhibitors. These intracellular signals typically elicit rapid
responses, and are important for the moment-to-moment regulation of metabolism.
B. Intercellular communication
The ability to respond to intercellular signals is essential for the development and survival of organisms. Signaling between cells provides for long-range integration of metabolism and usually results in a response, such as a change in gene expression, that is slower than is seen with intracellular signals. Communication between cells can be mediated, for example, by surface-to-surface contact and, in some tissues, by formation of gap junctions, allowing direct communication between the cytoplasms of adjacent cells. However, for energy metabolism, the most important route of communication is chemical signaling between cells by bloodborne hormones or by neurotransmitters.
C. Second messenger systems
Hormones or
neurotransmitters can be thought of as signals and their receptors as signal
detectors. Each component serves as a link in the communication between
extracellular events and chemical changes within the cell. Many receptors
signal their recognition of a bound ligand by initiating a series of reactions
that ultimately result in a specific intracellular response. “Second messenger”
molecules, so named because they intervene between the original messenger (the
neurotransmitter or hormone) and the ultimate effect on the cell, are part of
the cascade of events that translates (transduces) hormone or neurotransmitter
binding into a cellular response. Two of the most widely recognized second
messenger systems are the calcium/phosphatidylinositol system and the adenylyl
cyclase (adenylate cyclase) system, which is particularly important in
regulating the pathways of intermediary metabolism.
D. Adenylyl cyclase
The recognition of a
chemical signal by some plasma (cell) membrane receptors, such as the β- and
α2-adrenergic receptors, triggers either an increase or a decrease in the
activity of adenylyl cyclase (AC). This is a membrane-bound enzyme that
converts ATP to 3I ,5I -adenosine monophosphate (commonly
called cyclic AMP, or cAMP). The chemical signals are most often hormones or
neurotransmitters, each of which binds to a unique type of membrane receptor.
Therefore, tissues that respond to more than one chemical signal must have
several different receptors, each of which can be linked t o AC. These
receptors, known as G protein–coupled receptors (GPCRs), are characterized by
an extracellular ligand-binding domain, seven transmembrane α helices, and an
intracellular domain that interacts with G proteins (Figure 8.6).
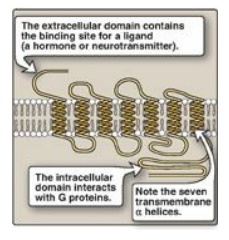
Figure 8.6 Structure of a
typical G protein-coupled receptor of the plasma membrane.
1. Guanosine triphosphate–dependent regulatory
proteins: The
effect of the activated, occupied GPCR on second messenger formation is not
direct but, rather, is mediated by specialized trimeric proteins (α, β, and γ
subunits) of the cell membrane. These proteins, referred to as G proteins
because the α subunit binds guanine nucleotides (GTP and GDP), form a link in
the chain of communication between the receptor and AC. In the inactive form of
a G protein, the a-subunit is bound to GDP (Figure 8.7). Binding of ligand
causes a conformational change in the receptor, triggering replacement of this
GDP with GTP. The GTP-bound form of the α subunit dissociates from the βγ
subunits and moves to AC, which is thereby activated. Many molecules of active
Gα protein are formed by one activated receptor. [Note: The ability of a
hormone or neurotransmitter to stimulate or inhibit AC depends on the type of
Gα protein that is linked to the receptor. One type, designated Gs, stimulates
AC, whereas another type, designated Gi, inhibits the enzyme (not shown in
Figure 8.7).] The actions of the Gα–GTP complex are short-lived because Gα has
an inherent GTPase activity, resulting in the rapid hydrolysis of GTP to GDP.
This causes inactivation of the Gα, its dissociation from AC, and reassociation
with the βγ dimer.
Toxins from Vibrio cholerae (cholera) and
Bordetella pertussis (whooping cough) cause inappropriate activation of
adenylyl cyclase through covalent modification (ADP-ribosylation) of different
G proteins. With cholera, the GTPase activity of Gαs is inhibited in intestinal
cells. With whooping cough, Gαi is inactivated in respiratory-tract cells.
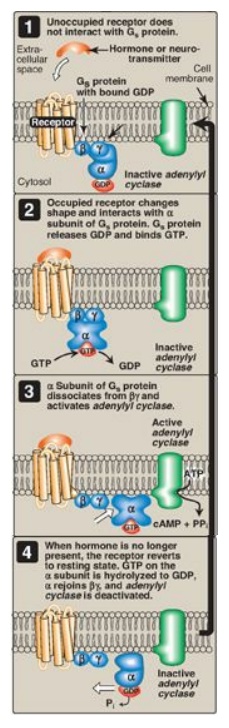
Figure 8.7 The recognition of chemical signals by certain membrane receptors triggers an increase (or, less often, a decrease) in the activity of adenylyl cyclase. GDP = guanosine diphosphate; GTP = guanosine triphosphate; cAMP = cyclic AMP.
2. Protein kinases: The next key link in the cAMP
second messenger system is the activation by cAMP of a family of enzymes called
cAMP-dependent protein kinases such as protein kinase A (Figure 8.8). cAMP
activates protein kinase A by binding to its two regulatory subunits, causing
the release of two active, catalytic subunits. The active subunits catalyze the
transfer of phosphate from ATP to specific serine or threonine residues of
protein substrates. The phosphorylated proteins may act directly on the cell’s
ion channels or, if enzymes, may become activated or inhibited. Protein kinase
A can also phosphorylate proteins that bind to DNA, causing changes in gene
expression. [Note: Several types of protein kinases are not cAMP dependent, for
example, protein kinase C.]
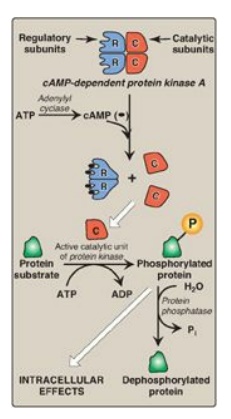
Figure 8.8 Actions of cyclic
AMP (cAMP). Pi = inorganic phosphate.
3. Dephosphorylation of proteins: The phosphate groups added to
proteins by protein kinases are removed by protein phosphatases, enzymes that
hydrolytically cleave phosphate esters (see Figure 8.8). This ensures that
changes in protein activity induced by phosphorylation are not permanent.
4. Hydrolysis of cyclic adenosine monophosphate: cAMP is rapidly hydrolyzed to 5 -AMP by cAMP phosphodiesterase, one of a family of enzymes that cleave the cyclic 3I ,5 I -phosphodiester bond. 5 I -AMP is not an intracellular signaling molecule. Therefore, the effects of neurotransmitter- or hormone-mediated increases of cAMP are rapidly terminated if the extracellular signal is removed. [Note: Phosphodiesterase is inhibited by the methylxanthine derivative, caffeine.]
Related Topics
