Classification and Structure of Carbohydrates
| Home | | Biochemistry |Chapter: Biochemistry : Introduction to Carbohydrates
Monosaccharides (simple sugars) can be classified according to the number of carbon atoms they contain.
CLASSIFICATION AND STRUCTURE
Monosaccharides (simple
sugars) can be classified according to the number of carbon atoms they contain.
Examples of some monosaccharides commonly found in humans are listed in Figure
7.1 . They can also be classified by the type of carbonyl group they contain. Carbohydrates
with an aldehyde as their carbonyl group are called aldoses, whereas those with
a keto as their carbonyl group are called ketoses (Figure 7.2 ). For example,
glyceraldehyde is an aldose, whereas dihydroxyacetone is a ketose.
Carbohydrates that have a free carbonyl group have the suffix –ose. [Note:
Ketoses have an additional “ul” in their suffix such as xyulose. There are
exceptions, such as fructose, to this rule.] Monosaccharides can be linked by
glycosidic bonds to create larger structures (Figure 7.3). Disaccharides
contain two monosaccharide units, oligosaccharides contain three to ten
monosaccharide units, and polysaccharides contain more than ten monosaccharide
units and can be hundreds of sugar units in length.
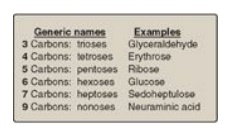
Figure 7.1 Examples of monosaccharides found in humans, classified according to the number of carbons they contain.
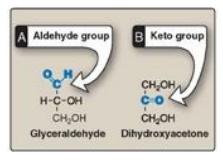
Figure 7.2 Examples of an
aldose (A) and a ketose (B) sugar.
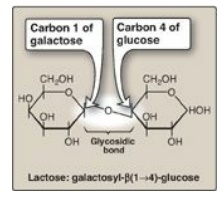
Figure 7.3 A glycosidic bond between two hexoses producing a disaccharide.
A. Isomers and epimers
Compounds that have the same chemical formula but have different structures are called isomers. For example, fructose, glucose, mannose, and galactose are all isomers of each other, having the same chemical formula, C6H12O6. Carbohydrate isomers that differ in configuration around only one specific carbon atom (with the exception of the carbonyl carbon; see “anomers” below) are defined as epimers of each other. For example, glucose and galactose are C-4 epimers because their structures differ only in the position of the –OH group at carbon 4. [Note: The carbons in sugars are numbered beginning at the end that contains the carbonyl carbon (that is, the aldehyde or keto group) as shown in Figure 7.4 .] Glucose and mannose are C-2 epimers. However, because galactose and mannose differ in the position of –OH groups at two carbons (carbons 2 and 4), they are isomers rather than epimers (see Figure 7.4).
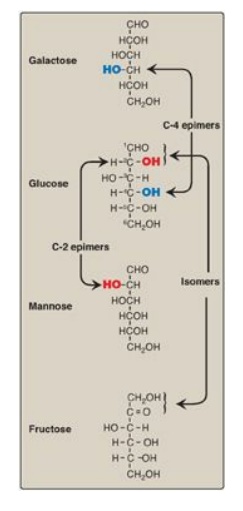
Figure 7.4 C-2 and C-4 epimers and an isomer of glucose.
B. Enantiomers
A special type of isomerism is found in the pairs of structures that are mirror images of each other. These mirror images are called enantiomers, and the two members of the pair are designated as a D- and an L-sugar (Figure 7.5). The vast majority of the sugars in humans are D-sugars. In the D isomeric form, the –OH group on the asymmetric carbon (a carbon linked to four different atoms or groups) farthest from the carbonyl carbon is on the right, whereas in the L-isomer, it is on the left. Most enzymes are specific for either the D or the L form, but enzymes known as racemases are able to interconvert D- and L-isomers.
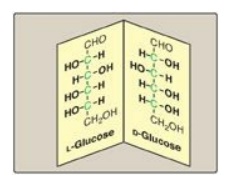
Figure 7.5 Enantiomers (mirror images) of glucose. Designation of D and L is by comparison to the triose, glyceraldehyde. [Note: The asymmetric carbons are shown in green.]
C. Cyclization of monosaccharides
Less than 1% of each of
the monosaccharides with five or more carbons exists in the open-chain
(acyclic) form in solution. Rather, they are predominantly found in a ring
(cyclic) form, in which the aldehyde (or keto) group has reacted with an
alcohol group on the same sugar, making the carbonyl carbon (carbon 1 for an
aldose, carbon 2 for a ketose) asymmetric. This asymmetric carbon is referred
to as the anomeric carbon.
1. Anomers: Creation of an anomeric carbon (the former carbonyl carbon), generates a new pair of isomers, the α and β configurations of the sugar (for example, α-D-glucopyranose and β-D-glucopyranose; see Figure 7.6 ), that are anomers of each other. [Note: In the α configuration, the –OH group on the anomeric carbon projects to the same side as the ring in a modified Fischer projection formula (Figure 7.6A ) and is trans to the CH2OH group in a Haworth projection formula (Figure 7.6B ). The α and β forms are not mirror images, and they are referred to as diastereomers.] Enzymes are able to distinguish between these two structures and use one or the other preferentially. For example, glycogen is synthesized from α-D-glucopyranose, whereas cellulose is synthesized from β-D-glucopyranose. The cyclic α and β anomers of a sugar in solution spontaneously (but slowly) form an equilibrium mixture, a process known as mutarotation (see Figure 7.6). [Note: For glucose, the α form makes up 36% of the mixture.]
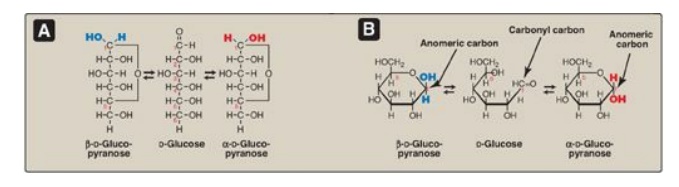
Figure 7.6 A The interconversion (mutarotation) of the α and β anomeric forms of glucose shown as modified Fischer projection formulas. B. The interconversion shown as Haworth projection formulas. [Note: A sugar with a six-membered ring (5C + 1O) is termed a pyranose, whereas one with a five-membered ring (4C + 1O) is a furanose. Virtually all glucose in solution is in the pyranose form.]
2. Reducing sugars: If the hydroxyl group on the
anomeric carbon of a cyclized sugar is not linked to another compound by a
glycosidic bond, the ring can open. The sugar can act as a reducing agent and
is termed a reducing sugar. Such sugars can react with chromogenic agents (for
example, the Benedict reagent) causing the reagent to be reduced and colored,
with the aldehyde group of the acyclic sugar becoming oxidized. All
monosaccharides, but not all disaccharides, are reducing sugars. [Note: Glucose
can have its terminal hydroxyl group oxidized to a carboxyl group, forming
glucuronic acid, or its aldehyde group oxidized to a hydroxyl group, forming a
sugar alcohol.]
A colorimetric test can detect a reducing sugar in urine. A positive result is indicative of an underlying pathology, because sugars are not normally present in urine, and can be followed up by more specific tests to identify the reducing sugar.
D. Joining of monosaccharides
Monosaccharides can be
joined to form disaccharides, oligosaccharides, and polysaccharides. Important
disaccharides include lactose (galactose + glucose), sucrose (glucose +
fructose), and maltose (glucose + glucose). Important polysaccharides include
branched glycogen (from animal sources) and starch (plant sources) and
unbranched cellulose (plant sources). Each is a polymer of glucose. The bonds
that link sugars are called glycosidic bonds. These are formed by enzymes known
as glycosyltransferases that use nucleotide sugars such as uridine diphosphate
glucose as substrates.
1. Naming glycosidic bonds: Glycosidic bonds between sugars
are named according to the numbers of the connected carbons and with regard to
the position of the anomeric hydroxyl group of the sugar involved in the bond.
If this anomeric hydroxyl is in the α configuration, the linkage is an α-bond.
If it is in the β configuration, the linkage is a β-bond. Lactose, for example,
is synthesized by forming a glycosidic bond between carbon 1 of β-galactose and
carbon 4 of glucose. The linkage is, therefore, a β(1→4) glycosidic bond (see
Figure 7.3 ). [Note: Because the anomeric end of the glucose residue is not
involved in the glycosidic linkage, it (and, therefore, lactose) remains a
reducing sugar.]
E. Complex carbohydrates
Carbohydrates can be attached by glycosidic bonds to noncarbohydrate structures, including purine and pyrimidine bases (found in nucleic acids), aromatic rings (such as those found in steroids and bilirubin), proteins (found in glycoproteins and proteoglycans), and lipids (found in glycolipids), to form glycosides.
1. N- and O-glycosides: If the group on the
noncarbohydrate molecule to which the sugar is attached is an –NH2
group, the structure is an N-glycoside, and the bond is called an N-glycosidic
link. If the group is an –OH, the structure is an O-glycoside, and the bond is
an O-glycosidic link (Figure 7.7 ). [Note: All sugar–sugar glycosidic bonds are
O-type linkages.]
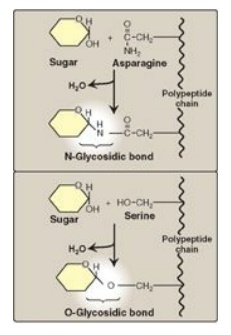
Figure 7.7 Glycosides:
examples of N- and O-glycosidic bonds.
Related Topics
