Chapter Summary, Study Questions
| Home | | Biochemistry |Chapter: Biochemistry : Bioenergetics and Oxidative Phosphorylation
The change in free energy (∆G) occurring during a reaction predicts the direction in which that reaction will spontaneously proceed.
CHAPTER SUMMARY
The change in free
energy (∆G) occurring during a reaction predicts the direction in which that
reaction will spontaneously proceed. If ∆G is negative (that is, the product
has a lower free energy than the substrate), the reaction goes spontaneously.
If ∆G is positive, the reaction does not go spontaneously. If ∆G = 0, the
reactions are in equilibrium. The ∆G of the forward reaction is equal in
magnitude but opposite in sign to that of the back reaction. The ∆Gs are
additive in any sequence of consecutive reactions, as are the standard free
energy changes (∆Gos). Therefore, reactions or processes that have a large,
positive ∆G are made possible by coupling with those that have a large,
negative ∆G such as hydrolysis of adenosine triphosphate (ATP). The reduced
coenzymes nicotinamide adenine dinucleotide (NADH) and flavin adenine
dinucleotide (FADH2) each donate a pair of electrons to a
specialized set of electron carriers, consisting of flavin mononucleotide (FMN)
, iron-sulfur centers, coenzyme Q, and a series of cytochromes, collectively
called the electron transport chain. This pathway is present in the inner
mitochondrial membrane (impermeable to most substances) and is the final common
pathway by which electrons derived from different fuels of the body flow to O2,
reducing it to water. The terminal cytochrome, cytochrome oxidase, is the only
cytochrome able to bind O2. Electron transport results in the
pumping of protons across the inner mitochondrial membrane from the matrix to
the intermembrane space. This process creates electrical and pH gradients
across the inner mitochondrial membrane. After protons have been transferred to
the cytosolic side of the membrane, they reenter the matrix by passing through
the Fo proton channel in ATP synthase (Complex V), dissipating the
pH and electrical gradients and causing conformational changes in the β
subunits of F1 that result in the synthesis of ATP from adenosine
diphosphate + inorganic phosphate. Electron transport and phosphorylation are
tightly coupled in oxidative phosphorylation (OXPHOS, Figure 6.17). Inhibition
of one process inhibits the other. These processes can be uncoupled by
uncoupling protein-1 of the inner mitochondrial membrane of cells in brown fat
and by synthetic compounds such as 2,4-dinitrophenol and aspirin, all of which
dissipate the proton gradient. In uncoupled mitochondria, the energy produced
by the transport of electrons is released as heat rather than being used to
synthesize ATP. Mutations in mitochondrial DNA, which is maternally inherited,
are responsible for some cases o f mitochondrial diseases such as Leber
hereditary optic neuropathy. The release of cytochrome c into the cytoplasm and
subsequent activation of proteolytic caspases results in apoptotic cell death.
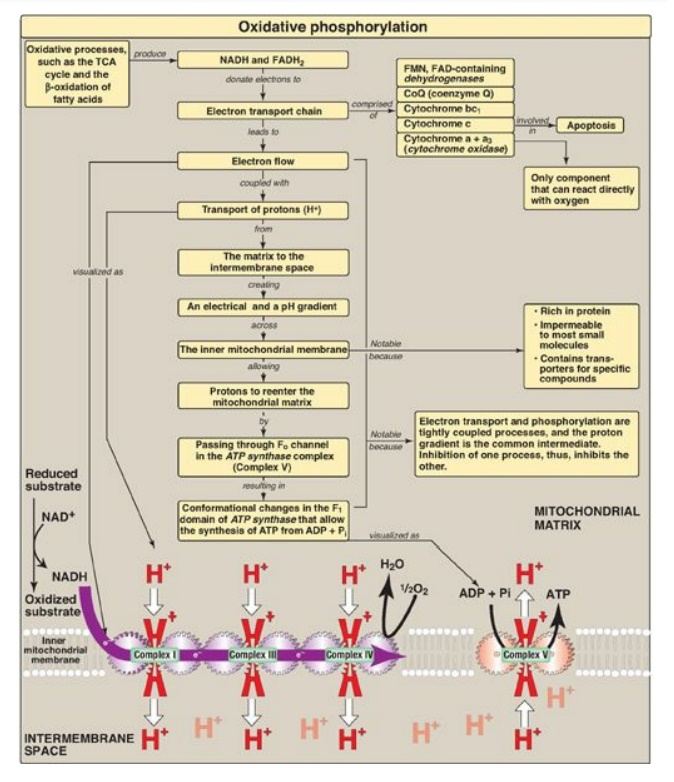
Figure 6.17 Key concept map for oxidative phosphorylation (OXPHOS). [Note: Electron (e-) flow and ATP synthesis are envisioned as sets of interlocking gears to emphasize the idea of coupling.] TCA = tricarboxylic acid; NAD(H) = nicotinamide adenine dinucleotide; FAD(H2) = flavin adenine dinucleotide; FMN = flavin mononucleotide.
Study Questions
Choose the ONE best answer.
6.1 2,4-Dinitrophenol, an uncoupler of oxidative
phosphorylation, was used as a weight-loss agent in the 1930s. Reports of fatal
overdoses led to its discontinuation in 1939. Which of the following would most
likely be true concerning individuals taking 2,4-dinitrophenol?
A. Adenosine
triphosphate levels in the mitochondria are greater than normal.
B. Body temperature is elevated as a result of
hypermetabolism.
C. Cyanide has no effect
on electron flow.
D. The proton gradient
across the inner mitochondrial membrane is greater than normal.
E. The rate of electron
transport is abnormally low.
Correct answer = B. When phosphorylation is uncoupled
from electron flow, a decrease in the proton gradient across the inner
mitochondrial membrane and, therefore, impaired ATP synthesis is expected. In
an attempt to compensate for this defect in energy capture, metabolism and
electron flow to oxygen is increased. This hypermetabolism will be accompanied
by elevated body temperature because the energy in fuels is largely wasted,
appearing as heat. The electron transport chain will still be inhibited by
cyanide.
6.2 Which of the following has the strongest
tendency to gain electrons?
A. Coenzyme Q
B. Cytochrome c
C. Flavin adenine
dinucleotide
D. Nicotinamide adenine
dinucleotide
E. Oxygen
Correct answer = E. Oxygen is the terminal acceptor of
electrons in the electron transport chain (ETC). Electrons flow down the ETC to
oxygen because it has the highest (most positive) reduction potential (E0).
The other choices precede oxygen in the ETC and have lower E0
values.
6.3 Explain why and how the malate-aspartate
shuttle moves nicotinamide adenine dinucleotide reducing equivalents from the
cytosol to the mitochondrial matrix.
There is no transporter
for nicotinamide adenine dinucleotide (NADH) in the inner mitochondrial
membrane. However, NADH can be oxidized to NAD+ by the cytoplasmic
isozyme of malate dehydrogenase as oxaloacetate is reduced to malate. The
malate is transported across the inner membrane, and the mitochondrial isozyme
of malate dehydrogenase oxidizes it to oxaloacetate as mitochondrial NAD+
is reduced to NADH. This NADH can be oxidized by Complex I of the electron
transport chain, generating three ATP through the coupled processes of
oxidative phosphorylation.
6.4 Carbon monoxide binds to and inhibits Complex
IV of the electron transport chain. What effect, if any, should this
respiratory inhibitor have on phosphorylation of adenosine diphosphate to
adenosine triphosphate?
Inhibition of the
electron transport chain by respiratory inhibitors such as carbon monoxide
results in an inability to maintain the proton gradient. Phosphorylation of ADP
to ATP is, therefore, inhibited, as are ancillary reactions such as calcium
uptake by mitochondria, because they also require the proton gradient.
Related Topics
