Phosphorylation of ADP to ATP
| Home | | Biochemistry |Chapter: Biochemistry : Bioenergetics and Oxidative Phosphorylation
The transfer of electrons down the ETC is energetically favored because NADH is a strong electron donor and O2 is an avid electron acceptor. However, the flow of electrons does not directly result in ATP synthesis.
PHOSPHORYLATION OF ADP TO ATP
The transfer of
electrons down the ETC is energetically favored because NADH is a strong
electron donor and O2 is an avid electron acceptor. However, the
flow of electrons does not directly result in ATP synthesis.
A. Chemiosmotic hypothesis
The chemiosmotic
hypothesis (also known as the Mitchell hypothesis) explains how the free energy
generated by the transport of electrons by the ETC is used to produce ATP from
ADP + Pi.
1. Proton pump: Electron transort is coupled to the
phosphorylation of ADP by the pumping of protons across the inner mitochondrial
membrane, from the matrix to the intermembrane space, at Complexes I, III, and
IV. This process creates an electrical gradient (with more positive charges on
the outside of the membrane than on the inside) and a pH gradient (the outside
of the membrane is at a lower pH than the inside) as shown in Figure 6.13. The
energy generated by this proton gradient is sufficient to drive ATP synthesis.
Thus, the proton gradient serves as the common intermediate that couples
oxidation to phosphorylation.
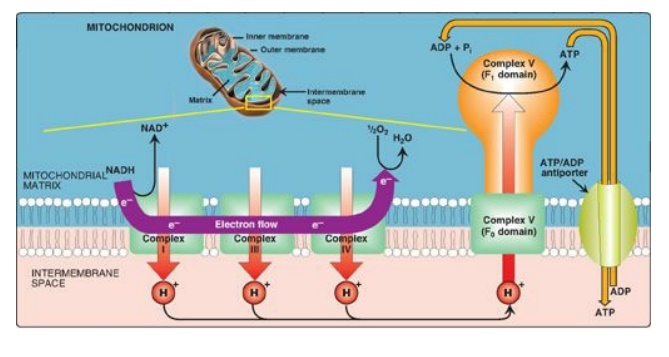
Figure 6.13 Electron transport chain shown in association with the transport of protons (H+). A total of ten H+ are pumped for each nicotinamide adenine dinucleotide (NADH) oxidized. [Note: H+ are not pumped at Complex II.]
2. ATP synthase: The multisubunit enzyme ATP synthase (Complex V;
see Figure 6.14) synthesizes ATP using the energy of the proton gradient. It
contains a domain (Fo) that spans the inner mitochondrial membrane,
and an extramembranous domain (F1) that appears as a sphere that
protrudes into the mitochondrial matrix (see Figure 6.13). The chemiosmotic
hypothesis proposes that after protons have been pumped to the cytosolic side
of the inner mitochondrial membrane, they reenter the matrix by passing through
a proton channel in the Fo domain, driving rotation of the c ring of
Fo and, at the same time, dissipating the pH and electrical gradients.
Fo rotation causes conformational changes in the β subunits of the F1
domain that allow them to bind ADP + Pi, phosphorylate ADP to ATP, and release
ATP. [Note: ATP synthase is also called F1/Fo-ATPase because the
isolated enzyme can catalyze the hydrolysis of ATP to ADP and Pi.]
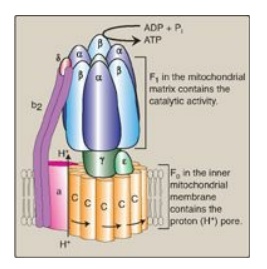
Figure 6.14 ATP synthase (F1Fo-ATPase). [Note: The rotation of the ring of c subunits in the Fo domain results in conformational changes in the β subunits of the F1 domain that allow phosphorylation of adenosine diphosphate (ADP) to adenosine triphosphate (ATP). Pi = inorganic phosphate.
a. Coupling in oxidative phosphorylation: In normal mitochondria, ATP synthesis
is coupled to electron transport through the proton gradient. Increasing (or
decreasing) one process has the same effect on the other. For example,
hydrolysis of ATP to ADP and Pi in energy-requiring reactions increases the
availability of substrates for ATP synthase and, thus, increases proton flow
through the enzyme. Electron transport and proton pumping by the ETC increase
to maintain the proton gradient. [Note: Increased oxidation of NADH at Complex
I and, consequently, an increase in NADH-producing pathways of metabolism, such
as the TCA cycle, results.]
b. Oligomycin: This drug binds to the Fo (hence the
letter “o”) domain of ATP synthase, closing the proton channel and preventing
reentry of protons into the matrix, thereby preventing phosphorylation of ADP
to ATP. Because the pH and electrical gradients cannot be dissipated in the
presence of this drug, electron transport stops because of the difficulty of
pumping any more protons against the steep gradients. This dependency of
cellular respiration on the ability to phosphorylate ADP to ATP is known as
respiratory control and is the consequence of the tight coupling of these
processes.
c. Uncoupling proteins: Uncoupling proteins (UCPs) occur
in the inner mitochondrial membrane of mammals, including humans. These
proteins form channels that allow protons to reenter the mitochondrial matrix
without energy being captured as ATP (Figure 6.15). The energy is released as
heat, and the process is called nonshivering thermogenesis. UCP1, also called thermogenin,
is responsible for heat production in the brown adipocytes of mammals. In brown
fat, unlike the more abundant white fat, almost 90% of its respiratory energy
is used for thermogenesis in response to cold in the neonate and during arousal
in hibernating animals. However, humans appear to have few concentrated
deposits of brown fat (except in the newborn), and UCP1 does not appear to play
a major role in energy balance. [Note: Uncoupling proteins UCP2–UCP5 have been
found in other tissues, but their full significance remains unclear.]
d. Synthetic uncouplers: Electron transport and
phosphorylation can also be uncoupled by compounds that pick up protons in the
intermembrane space and release them in the matrix, dissipating the gradient.
The classic example is 2,4-dinitrophenol, a lipophilic proton carrier that
readily diffuses through the mitochondrial membrane. This uncoupler causes
electron transport to proceed at a rapid rate without establishing a proton
gradient, much as do the UCPs (see Figure 6.15). Again, energy is released as
heat rather than being used to synthesize ATP. [Note: In high doses, aspirin
and other salicylates uncouple oxidative phosphorylation. This explains the
fever that accompanies toxic overdoses of these drugs.]
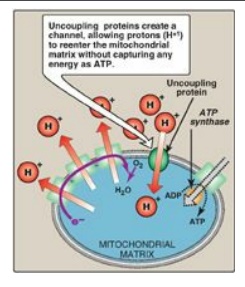
Figure 6.15 Transport of
protons across the mitochondrial membrane by an uncoupling protein. ADP =
adenosine diphosphate; ATP = adenosine triphosphate.
B. Membrane transport systems
The inner mitochondrial
membrane is impermeable to most charged or hydrophilic substances. However, it
contains numerous transport proteins that permit passage of specific molecules
from the cytosol (or more correctly, the intermembrane space) to the
mitochondrial matrix.
1. ATP and ADP transport: The inner membrane requires
specialized carriers to transport ADP and Pi from the cytosol (where ATP is
hydrolyzed to ADP in many energy-requiring reactions) into mitochondria, where
ATP can be resynthesized. An adenine nucleotide antiporter imports one ADP from
the cytosol into the matrix, while exporting one ATP from the matrix into the
cytosol (see Figure 6.13). A transporter moves Pi from the cytosol into
mitochondria.
2. Transport of reducing equivalents: The inner mitochondrial membrane
lacks an NADH transporter, and NADH produced in the cytosol (for example, in
glycolysis;) cannot directly enter the mitochondrial matrix. However, two
electrons (reducing equivalents) of NADH are transported from the cytosol into
the matrix using substrate shuttles. In the glycerophosphate shuttle (Figure
6.16A), two electrons are transferred from NADH to dihydroxyacetone phosphate
by cytosolic glycerophosphate dehydrogenase. The glycerol 3-phosphate produced
is oxidized by the mitochondrial isozyme as FAD is reduced to FADH2.
CoQ of the ETC oxidizes the FADH2. The glycerophosphate shuttle,
therefore, results in the synthesis of two ATPs for each cytosolic NADH
oxidized. This contrasts with the malate-aspartate shuttle (Figure 6.16B), which
produces NADH (rather than FADH2 FADH2 FADH2 FADH2) in the
mitochondrial matrix and, therefore, yields three ATPs for each cytosolic NADH
oxidized by malate dehydrogenase as oxaloacetate is reduced to malate. A
transport protein moves malate into the matrix.
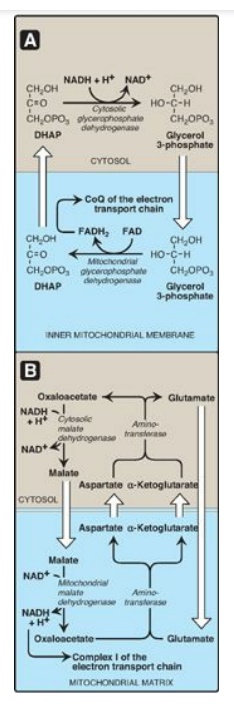
Figure 6.16 Substrate shuttles for the transportof electrons across the inner mitochondrial membrane. A. Glycerophosphate shuttle. B. Malate-aspartate shuttle. DHAP = dihydroxyacetone phosphate; NAD(H) = nicotinamide adenine dinucleotide; FAD(H2) = flavin adenine dinucleotide; CoQ = coenzyme Q.
C. Inherited defects in oxidative phosphorylation
Thirteen of the
approximately 90 polypeptides required for oxidative phosphorylation are coded
for by mtDNA and synthesized in mitochondria, whereas the remaining proteins
are coded for by nuclear DNA, synthesized in the cytosol, and transported into mitochondria
posttranslationally. Defects in oxidative phosphorylation are more likely a
result of alterations in mtDNA, which has a mutation rate about 10 times
greater than that of nuclear DNA. Tissues with the greatest ATP requirement
(for example, central nervous system, skeletal and heart muscle, and liver) are
most affected by defects in oxidative phosphorylation. Mutations in mtDNA are
responsible for several diseases, including some cases of mitochondrial
myopathies, and Leber hereditary optic neuropathy, a disease in which bilateral
loss of central vision occurs as a result of neuroretinal degeneration,
including damage to the optic nerve. [Note: mtDNA is maternally inherited
because mitochondria from the sperm cell do not enter the fertilized egg.]
D. Mitochondria and apoptosis
The process of apoptosis, or programmed cell death, may be initiated through the intrinsic (mitochondrial-mediated) pathway by the formation of pores in the outer mitochondrial membrane. These pores allow cytochrome c to leave the intermembrane space and enter the cytosol. There, cytochrome c, in association with proapoptotic factors, activates a family of proteolytic enzymes (the caspases), causing cleavage of key proteins and resulting in the morphologic and biochemical changes characteristic of apoptosis.
Related Topics
