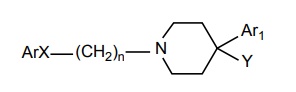
SAR of Butyrophenones
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Tranquillizers
Antipsychotic activity is seen when Ar group is an aromatic system in which fluoro-substituents at para-position enhances the activity.
SAR of Butyrophenones Antipsychotic activity is seen when Ar group is an aromatic system in which fluoro-substituents at para-position enhances the activity. When X = carbonyl ( C = O) optimal activity is seen, although other groups C(H)OH, C(H) aryl (pimozide) also afford good activity. When n = 3 activity is optimal, longer, or shorter chains decrease the activity. Aliphatic amino nitrogen is required and highest activity is seen when it is incorporated into a cyclic form Ar1 group should be an aromatic and is needed; it should be attached directly to the fourth position or occasionally separated from it by one intervening atom. The Y group can vary and assist the activity. The empirical SARs could be constructed to suggest that the 4-aryl piperidino moiety is super imposable on the 2-phenyl ethyl amino moiety of dopamine and accordingly could promote affinity for D2 and D3 receptors. The long N-alkyl substituent could help to promote receptor affinity and produce receptor antagonism activity (or) inverse agonism.