Static Beds of Nonporous Solids
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Drying
Drying of wet granular beds, the particles of which are not porous and are insoluble in the wetting liquid, has been extensively studied.
STATIC BEDS OF NONPOROUS SOLIDS
Drying
of wet granular beds, the particles of which are not porous and are insoluble
in the wetting liquid, has been extensively studied. The operation is presented
as the relation of moisture content and time of drying in Figure 7.3A. It
should be noted that the equilibrium moisture content is approached slowly. A
protracted period may be required for the removal of water just above the equilibrium
value. This is not justified if a small amount of water can be tol-erated in
further processing and indicates the importance of establishing
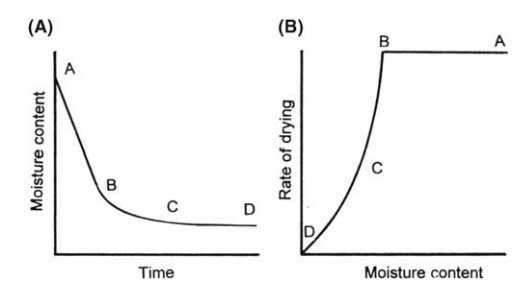
FIGURE 7.3 (A) Moisture content versus
time of drying and (B) rate of drying versus moisture content.
The stability of the solids, maintained, as shown later, at a
temperature close to that of the drying air, may allow unnecessary
deterioration.
The data has been
converted to a curve relating the rate of drying to moisture content in Figure
7.3B. The initial heating-up period during which equilibrium is established is
short and has been omitted from both figures.
Assuming that
sufficient moisture is initially present, the drying rate curve exhibits three
distinct sections limited by the points A, B, C, and D.
In section AB,
called the constant rate period, it
is considered that moisture is evaporating from a saturated surface at a rate
governed by diffusion from the surface through the stationary air film in
contact with it. An analogy with evaporation from a plain water surface can
therefore be drawn. The rate of drying during this period depends on the air
temperature, humidity, and speed, which in turn determine the temperature of
the saturated surface. Assuming that these are constant, all variables in the
drying equations given above are fixed and a constant rate of drying is
established, which is largely independent of the material being dried. The
drying rate is somewhat lower than that for a free water surface and depends to
some extent on the particle size of the solids. During the constant rate
period, liquid must be transported to the surface at a rate sufficient to
maintain saturation. The mechanism of transport is discussed later.
At the end of the
constant rate period, B, a break in the drying curve occurs. This point is
called the critical moisture content, and a linear fall in the drying rate
occurs with further drying. This section, BC, is called the first falling rate
period. At and below the critical moisture content, the movement of moisture
from the interior is no longer sufficient to saturate the surface. As drying
pro-ceeds, moisture reaches the surface at a decreasing rate and the mechanism
that controls its transfer will influence the rate of drying. Since the surface
is no longer saturated, it will tend to rise above the wet bulb temperature.
For
any material, the critical moisture content decreases as the particle size
decreases. Eventually, moisture does not reach the surface that becomes dry.
The plane of evaporation recedes into the solid, the vapor reaching the surface
by diffusion through the pores of the bed. This section is called the second
falling rate period and is controlled by vapor diffusion, a factor which will be
largely
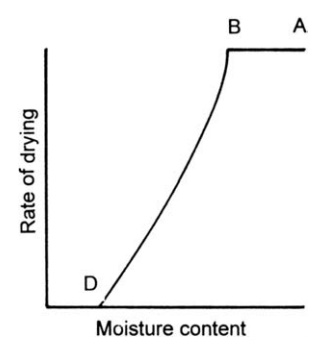
FIGURE 7.4 Drying curve for a skin-forming
material.
independent
of the conditions outside the bed but markedly affected by the particle size
because of the latter’s influence on the dimensions of pores and channels.
During this period, the surface temperature approaches the temper-ature of the
drying air.
Considerable
migration of liquid occurs during the constant rate and first falling rate
periods. Associated with the liquid will be any soluble constituents that will
form a concentrating solution in the surface layers as drying proceeds.
Deposition of these materials will take place when the surface dries.
Consider-able segregation of soluble elements in the cake can, therefore, occur
during drying.
If
the soluble matter forms a skin or gel on drying rather than a crystalline
deposit, a different drying curve, shown in Figure 7.4, is obtained. The
constant rate period is followed by a continuous fall in the drying rate in
which no differentiation of first and second falling rate periods can be made
(hence “C” is not identified in Fig. 7.4). During this period, drying is
controlled by diffusion through the skin that is continually increasing in
thickness. Soap and gelatin are solutes that behave in this way.
Related Topics
