Status of Oral Hypoglycaemics in Diabetes Mellitus
| Home | | Pharmacology |Chapter: Essential pharmacology : Insulin, Oral Hypoglycaemic Drugs and Glucagon
After 8 years of prospective study involving large number of patients, the University Group Diabetes Programme (UGDP) of USA (1970) presented findings that cardiovascular mortality was higher in patients treated with oral hypoglycaemics than in those treated with diet and exercise alone or with insulin.
STATUS OF ORAL HYPOGLYCAEMICS IN DIABETES MELLITUS
After 8 years of
prospective study involving large number of patients, the University Group
Diabetes Programme (UGDP) of USA (1970) presented findings that cardiovascular
mortality was higher in patients treated with oral hypoglycaemics than in those
treated with diet and exercise alone or with insulin. A decline in their use followed.
Subsequent studies both refuted and supported these conclusions.
The controversy has
now been settled; UK PDS found that both sulfonylureas and metformin did not
increase cardiovascular mortality over > 10 years observation period.
Related to degree of glycaemia control, both insulin and sulfonylureas reduced
microvascular complications in type 2 DM, but did not have significant effect
on macrovascular complications. Metformin, however, could reduce macrovascular
complications as well; it decreased risk of death and other diabetes related
endpoints in overweight patients. This may be related to the fact that both
sulfonylureas and exogenous insulin improve glycaemic control by increasing
insulin supply rather than by reducing insulin resistance, while metformin can
lower insulin resistance. The thiazolidinediones are another class of drugs
which reverse insulin resistance, and have been found to reduce macrovascular
complications and mortality in type 2 DM. All oral hypoglycaemics do however
control symptoms that are due to hyperglycaemia and glycosuria, and are much
more convenient than insulin.
Oral hypoglycaemics
are indicated only in type 2 diabetes, when not controlled by diet and
exercise. They are best used in patients with—
i.
Age above 40 years at onset of disease.
ii.
Obesity at the time of presentation.
iii.
Duration of disease < 5 years when starting
treatment.
iv.
Fasting blood sugar < 200 mg/dl.
v.
Insulin requirement < 40 U/day.
vi.
No ketoacidosis or a history of it, or any
other complication.
Introduced in the
prediabetic ‘impaired glucose tolerance phase’, sulfonylurea + dietary regulation
has been shown to postpone manifest type 2 DM. This may be due to the fact that
hyperglycaemia is a self perpetuating condition. The Diabetes Prevention Programme
(2002) has established that in middle aged, obese prediabetics metformin
prevented progression to type 2 DM, but not in older nonobese prediabetics.
Glitazones appear to have similar potential. Longterm acarbose therapy can also
prevent type 2 DM.
Oral hypoglycaemics
should be used to supplement dietary management and not to replace it.
Metformin is preferred in obese type 2 patients: its anorectic action aids
weight reduction and it has the potential to lower risk of myocardial
infarction and stroke. The g.i. tolerance of metformin is poorer and its
patient acceptability is less than that of sulfonylureas. Moreover, the
sulfonylureas appear to produce greater blood sugar lowering. As such, many
patients are treated initially with a sulfonylurea alone. Metformin can be used
to supplement sulfonylureas in patients not adequately controlled by the
latter.
There is no difference
in the clinical efficacy of different sulfonylureas. This however does not
signify that choice of drug is irrelevant. Differences between them are mainly
in dose, onset and duration of action which governs flexibility of regimens. The
second generation drugs are dose to dose more potent, produce fewer side
effects and drug interactions, and are commonly used, but no spectacular features
have emerged.
Chlorpropamide is not recommended
because of long duration of action,
greater risk of hypoglycaemia, jaundice, alcohol flush, dilutional hyponatraemia
and other adverse effects. Tolbutamide
is less popular due to low potency, but may be employed in the elderly to avoid
hypoglycaemia. Glibenclamide and glyclazide are suitable for most patients, but have been found to cause
hypoglycaemia more frequently. Glipizide
is preferred when a faster and shorter acting drug is required. Glimepiride is a newer sulfonylurea,
claimed to have stronger
extrapancreatic action by enhancing GLUT4 translocation to the plasma membrane,
thus causing lesser hyperinsulinaemia.
A low incidence of
hypoglycaemic episodes has been reported with glimepiride. This may be due to
its ability to preserve hypoglycaemia induced glucagon release and suppression
of insulin release, responses that are attenuated by glibenclamide. Glimepiride
is suitable for once daily dosing due to gradual release from tissue binding.
Even in properly selected
patients, sulfonylureas may fail from the beginning (primary failure 5–28%) or
become ineffective after a few months or years of satisfactory control (secondary
failure 5–10% per year): may be due to progressive loss of β cells, reduced
physical activity, continuing insulin resistance, drug and dietary
noncompliance or desensitization of receptors. If one sulfonylurea proves
ineffective in a patient, another one (especially a second generation) may still
work. Combined use of a sulfonylurea and a biguanide may be tried if either is
not effective alone and the glitazones are now available as add on/alternative
drugs. Patients with marked/only posprandial hyperglycaemia may be treated with
repaglinide/ nateglinide. Upto 50% patients of type 2 DM initially treated with
oral hypoglycaemics ultimately need insulin. Despite their limitations, oral
hypoglycaemics are suitable therapy for majority of type 2 DM patients.
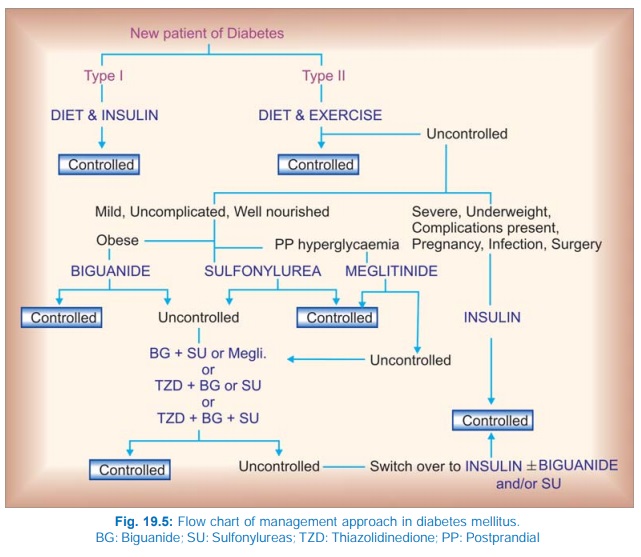
However, when a diabetic on oral hypoglycaemics presents with infection, severe
trauma or stress, pregnancy, ketoacidosis or any other complication or has to
be operated upon—switch over to insulin (see
Fig. 19.5).
Sulfonylureas and
metformin can also be combined with insulin, particularly when a single daily
injection of longacting insulin is used to provide basal control, the oral
hypoglycaemics given before meals serve to check postprandial glycaemia.
Guargum
It
is a dietary fibre (polysaccharide) from Indian cluster beans
(Guar), which forms a viscous gel on contact with water. Administered just
before or mixed with food, it slows gastric emptying, intestinal transit and
carbohydrate absorption: postprandial glycaemia is suppressed but overall
lowering of blood glucose is marginal. It also reduces serum cholesterol by
about 10%.
Guargum can be used to supplement diet and to lower sulfonylurea
dose, and as a hypocholesterolemic. It is not absorbed but fermented in the
colon. Side effects are flatulence, feeling of fullness, loss of appetite,
nausea, gastric discomfort and diarrhoea. Start with a low dose (2.5 g/day) and
gradually increase to 5 g TDS.
DIATAID, CARBOTARD 5 g
sachet.
Glucomannan
This
powdered extract from tubers of Konjar is promoted as
a dietary adjunct for diabetes. It swells in the stomach by absorbing water and
is claimed to reduce appetite, blood sugar, serum lipids and relieve
constipation.
DIETMANN 0.5 g cap, 1
g sachet; 1 g to be taken before meals.
