Synthetic Reactions
| Home | | Pharmacology |Chapter: Essential pharmacology : Pharmacokinetics; Metabolism Excretion Of Drugs, Kinetics Of Elimination
These involve conjugation of the drug or its phase I metabolite with an endogenous substrate, generally derived from carbohydrate or amino acid, to form a polar highly ionized organic acid, which is easily excreted in urine or bile. Conjugation reactions have high energy requirement.
SYNTHETIC REACTIONS
These involve conjugation
of the drug or its phase I metabolite with an endogenous substrate, generally
derived from carbohydrate or amino acid, to form a polar highly ionized organic
acid, which is easily excreted in urine or bile. Conjugation reactions have
high energy requirement.
i) Glucuronide Conjugation
This is the most important synthetic reaction
carriedout by a group of UDPglucuronosyl transferases (UGTs). Compounds with a
hydroxyl or carboxylic acid group are easily conjugated with glucuronic acid
which is derived from glucose. Examples are— chloramphenicol, aspirin,
paracetamol, lorazepam, morphine, metronidazole. Not only drugs but endogenous
substrates like bilirubin, steroidal hormones and thyroxine utilize this
pathway. Glucuronidation increases the molecular weight of the drug which
favours its excretion in bile. Drug glucuronides excreted in bile can be
hydrolysed by bacteria in the gut—the liberated drug is reabsorbed and
undergoes the same fate. This enterohepatic cycling of the drug prolongs its
action, e.g. phenolphthalein, oral contraceptives.
ii)
Acetylation
Compounds having amino or hydrazine residues are
conjugated with the help of acetyl coenzymeA, e.g. sulfonamides, isoniazid,
PAS, hydralazine, clonazepam, procainamide. Multiple genes control the Nacetyl
transferases (NATs), and rate of acetylation shows genetic polymorphism (slow
and fast acetylators).
iii)
Methylation
The amines and phenols can be methylated; methionine and cysteine acting
as methyl donors, e.g. adrenaline, histamine, nicotinic acid, methyldopa,
captopril, mecarptopurine.
iv) Sulfate Conjugation
The phenolic compounds and steroids are sulfated by sulfotransferases
(SULTs), e.g. chloramphenicol, methyldopa, adrenal and sex steroids.
iv)
Glycine conjugation
Salicylates and other drugs having carboxylic acid group are
conjugated with glycine, but this is not a major pathway of metabolism.
vi) Glutathione conjugation
Forming a mercapturate is normally a minor pathway.
However, it serves to inactivate highly reactive quinone or epoxide intermediates
formed during metabolism of certain drugs, e.g. paracetamol. When large amount
of such intermediates are formed (in poisoning or after enzyme induction),
glutathione supply falls short—toxic adducts are formed with tissue
constituents → tissue damage.
vii) Ribonucleoside/nucleotide synthesis
This pathway is important
for the activation of many purine and pyrimidine antimetabolites used in cancer
chemotherapy.
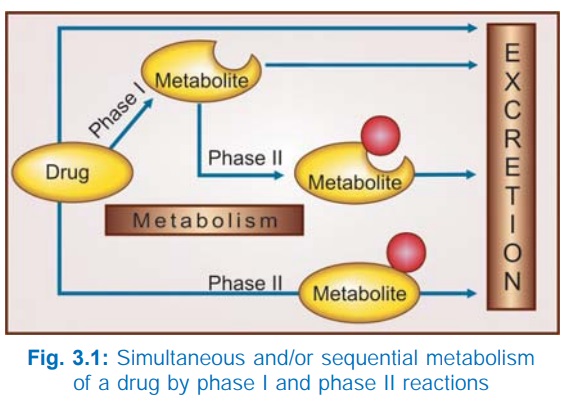
Most drugs are
metabolized by many pathways, simultaneously or sequentially as illustrated in
Fig. 3.1. Rates of reaction by different pathways often vary considerably. A
variety of metabolities (some more, some less) of a drug may be produced.
Stereoisomers of a drug may be metabolized differently and at different rates,
e.g. Swarfarin rapidly undergoes ring oxidation, while Rwarfarin is slowly
degraded by sidechain reduction.
Only a few drugs are metabolized by enzymes of
intermediary metabolism, e.g. alcohol by dehydrogenase, allopurinol by xanthine
oxidase, succinylcholine and procaine by plasma cholinesterase, adrenaline by
monoamine oxidase. Majority of drugs are acted on by relatively nonspecific
enzymes which are directed to types of molecules rather than to specific drugs.
The same enzyme can metabolize many drugs. The drug metabolising enzymes are
divided into two types:
Microsomal Enzymes
These are located on smooth endoplasmic reticulum (a system of
microtubules inside the cell), primarily in liver, also in kidney, intestinal
mucosa and lungs. The monooxygenases, cytochrome P 450, glucuronyl transferase,
etc. are microsomal enzymes.
They catalyse most of the oxidations, reductions,
hydrolysis and glucuronide conjugation. Microsomal enzymes are inducible by
drugs, diet and other agencies.
Nonmicrosomal Enzymes
These are present in the cytoplasm and mitochondria
of hepatic cells as well as in other tissues including plasma. The flavoprotein
oxidases, esterases, amidases and conjugases are nonmicrosomal. Reactions
catalysed are:
Some oxidations and reductions, many hydrolytic
reactions and all conjugations except glucuronidation.
The nonmicrosomal enzymes are not inducible
but many show genetic polymorphism (acetyl transferase, pseudocholinesterase).
Both microsomal and nonmicrosomal enzymes are
deficient in the newborn, especially premature, making them more susceptible to
many drugs, e.g. chloramphenicol, opioids. This deficit is made up in first few
months, more quickly in case of oxidation and other phase I reactions than in
case of glucuronide and other conjugations taking 3 or more months.
The
amount and kind of drug metabolizing enzymes is controlled genetically and is
also altered by environmental factors. Thus, marked interspecies and
interindividual differences are seen, e.g. cats are deficient in glucuronyl transferase
while dogs are deficient in acetyl transferase. Upto 6fold difference in the
rate of metabolism of a drug among normal human adults may be observed. This is
one of the major causes of individual variation in drug response.
