The Role of Drug Metabolism in Type B Adverse Drug Reactions
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Mechanisms of Adverse Drug Reactions
TYPE B OR IDIOSYNCRATIC ADVERSE DRUG REACTIONS
THE ROLE OF DRUG METABOLISM IN
TYPE B ADVERSE DRUG REACTIONS
In
general, drug metabolism can be considered to be a detoxication process in that
it converts thera-peutically active compounds to inactive metabolites, which
can then be excreted harmlessly from the body. This process may require one or
more than one drug-metabolising enzyme that may be a phase I and/or II enzyme
(Woolf and Jordan, 1987) (Figure 8.2). A drug may undergo sequential phases I
and II metabolism, or alternatively, it may only undergo either phase I or
phase II metabolism (Tephly and Burchell, 1990).
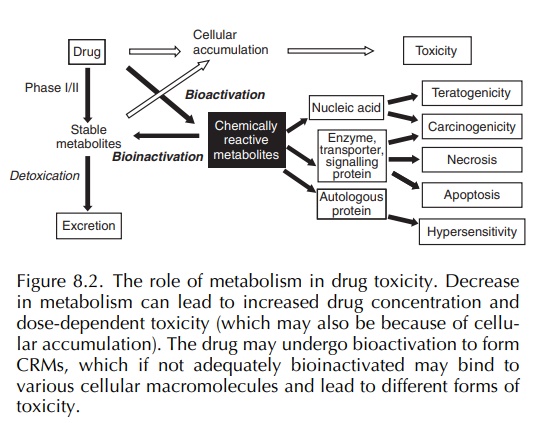
In certain circumstances, the drug-metabolising enzymes can convert a drug to a toxic, CRM, a process termed ‘bioactivation’ (Pirmohamed, Kitteringham and Park, 1994; Pirmohamed, Madden and Park, 1996) (Figure 8.2). Bioactivation may represent less than 1% of the overall metabolism of a drug. The body is equipped with formidable defence mecha-nisms, and in most cases, the CRM will be detoxified (a process which can be termed ‘bioinactivation’) before it can initiate tissue damage. Indeed, it is possible that most therapeutically used drugs undergo some degree of bioactivation but do not cause toxi-city because the amount of toxic metabolite formed is below a ‘toxic’ threshold or it is promptly detox-ified. Both phases I and II enzymes can cause drug bioactivation, but in most cases, it is the former, i.e. the cytochrome P450 enzymes, which are responsible (Pirmohamed, Kitteringham and Park, 1994).
Inadequate
detoxication of a CRM is often the first step in the initiation of
idiosyncratic drug toxicity (Park, Pirmohamed and Kitteringham, 1992;
Pirmohamed, Kitteringham and Park, 1994). This may occur if there is an imbalance
between drug bioactivation and bioin-activation pathways. Tissue-specific
expression of enzymes involved in drug bioactivation and drug detox-ication may
lead to a selective imbalance in that tissue resulting in tissue-selective
toxicity (Park, Pirmohamed and Kitteringham, 1995). An imbalance may be the
consequence of a genetically determined deficiency of an enzyme, or
alternatively, it may be acquired because of environmental factors such as
infection, diet or concomitant drug intake. It is important to note that
inadequate detoxication of a CRM, although an impor-tant first step in the
occurrence of toxicity, is not neces-sarily the ultimate step (Pirmohamed,
Madden and Park, 1996). Other factors such as tissue repair enzymes, immune
responsiveness and the biochemical processes that modulate tissue injury may
all serve as factors determining not only whether idiosyncratic toxicity occurs
but also its severity.
An
inadequately detoxified CRM can combine with or damage cellular macromolecules
such as proteins and nucleic acids and result in various forms of toxi-city
including teratogenicity, carcinogenicity, cellular necrosis and
hypersensitivity (Park, Pirmohamed and Kitteringham, 1995) (Figure 8.2). The
binding of a CRM to nucleic acid may result in teratogenicity or
carcinogenicity (Figure 8.2).
The binding to cellular macromolecules may result in either direct or immune-mediated toxicity (Pirmohamed, Kitteringham and Park) (Figure 8.2). With direct toxicity, binding of the CRM to a protein will interfere with its normal physiological function leading to cellular necrosis. Alternatively, the CRM can act as a hapten and initiate an immune reac-tion that may be because of a specific humoral (antibody) response, a cellular response (T lympho-cytes) or a combination of both (Naisbitt et al., 2000a; Park, Coleman and Kitteringham, 1987; Park et al., 2001; Park, Pirmohamed and Kitteringham, 1998; Pohl et al., 1988). The immune response can be directed against the drug (haptenic epitopes), the carrier protein (auto-antigenic determinants) or the neoantigen created by the combination of the drug and the protein (new antigenic determinants). The factors that determine what type of toxicity is medi-ated by a CRM are poorly understood but are likely to include (Boelsterli, 1993; Gillette, Lau and Monks, 1984; Park, Coleman and Kitteringham, 1987)
• the relative stability of the CRM, and thus its reactivity;
• the half-life of any drug–protein adducts that are formed and their concentration within the cell;
• the epitope density, i.e. the number of groups of the CRM that are covalently bound to a protein molecule; and
• the nature, physiological function and subcellular site of the carrier protein to which the CRM binds.
In most cases, the differentiation between these two forms of idiosyncratic toxicity is largely empirical being based on symptomatology; e.g. the occurrence of manifestations such as rash, fever, lymphadenopa-thy and eosinophilia all suggest drug hypersensitivity (Pessayre and Larrey, 1988; Pirmohamed et al., 1998). The lack of laboratory methodology by which to make a definitive diagnosis largely reflects our ignorance of the mechanism of toxicity in most cases of idiosyn-cratic toxicity.
Related Topics
