Using MedDRA for Data Entry
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Medical Dictionary for Regulatory Activities (MedDRA)
The process of coding adverse event or other medi-cal information with MedDRA involves the use of computer software: either a ‘browser’ or an ‘autoencoder’.
USING MedDRA FOR DATA ENTRY
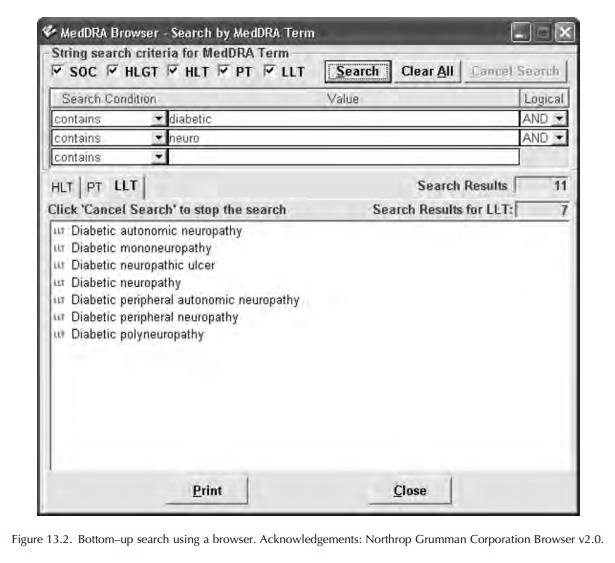
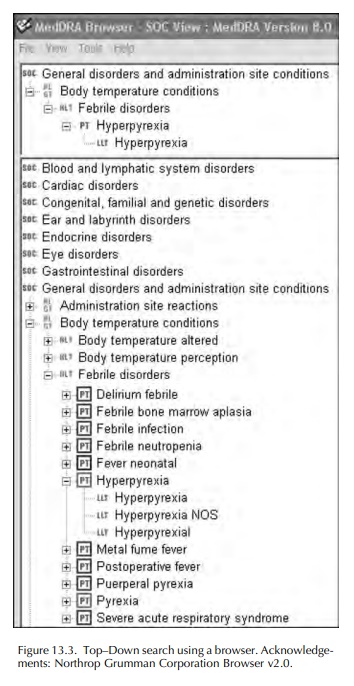
The process of coding adverse event or other medi-cal information with MedDRA involves the use of computer software: either a ‘browser’ or an ‘autoencoder’. These are available commercially, or a browser may be downloaded from the MSSO website. A browser permits the user to search MedDRA for an LLT to match the verbatim or ‘as reported’ term. Most browsers provide some type of Boolean search facility, with ‘and/or’ commands, or the possibility to search for LLTs beginning with, or containing, selected parts of words. Many browsers also present a view of the MedDRA ‘tree’ and enable this to be searched starting with the SOC likely to contain the concept being searched, then drilling down through the HLGT, HLT and PT until appropriate LLTs can be viewed and selected. An illustration of the appearance of MedDRA using a browser is shown in Figures 13.2 and 13.3.
Autoencoders
may have the additional capability of scanning narrative texts and presenting
expressions likely to need coding. They will often store selec-tions of LLTs
that closely match verbatim terms coded historically, in order to improve
consistency of term selection. They can code long lists of verbatim terms,
presenting the user with a list of identical or closely matching LLTs that can
then be confirmed as being acceptable or rejected.
Guidelines
on the selection of terms used to code adverse events have been published by
the MSSO (MedDRA® Term Selection, 2004), with the endorse-ment of
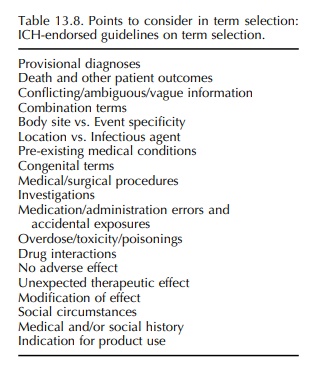
ICH. These ‘Points to Consider’ guidelines cover the topics shown in Table
13.8. It is important that each MedDRA subscriber has its own written procedures
that are consistent with these guidelines, in order to make coding as
consistent as possible across the organisation concerned.
The
general principles presented in the ICH-endorsed guidelines are as follows:
1. Try to clarify
ambiguous, confusing or unintelli-gible data.
2. Promote quality
through form design and by train-ing those involved in collection and
follow-up.
3. Select the LLT that
most accurately reflects the reporter’s words.
4. Use current LLTs
only (unless for legacy data conversion).
5. Use medical
judgement if there is no exact match for a verbatim term, if there is an
existing adequate representation in MedDRA.
6. It is not
appropriate to address deficiencies in MedDRA by developing
organisation-specific solutions.
7. If there is no
adequate representation of a concept in MedDRA, submit a change request to the
MSSO.
8. If a specific
medical concept (e.g. metastatic colon cancer) has no single MedDRA term,
request a new term, and in the interim, use one or more existing terms (e.g. Colon cancer, or Metas-tases or use the two terms
Colon cancer and Metastases)
9. Do not subtract or
add information: no medical concepts should be excluded from coding; code
regardless of causality assessment.
10. Do not invent diagnoses or mechanisms: use the information as provided by the reporter.
11.Documentation of selection strategies and Quality
Assurance procedures are encouraged.
12.Human intervention is essential to ensure that the end
result reflects the original information and makes medical sense.
13. Do not make ad
hoc structural changes to
MedDRA: the assignment
of SOCs is pre-determined and should not be altered
by users, although a change request may be made if terms are incorrectly placed.
The
guidelines also suggest that if a report of an adverse event includes a
diagnosis and its symptoma-tology, it is sufficient to select a term for the
diagnosis and not for the signs and symptoms. It remains an option to code the
signs and symptoms in addition. If there are signs and symptoms that are not
usually part of the diagnosis, these should be coded as well as the diagnosis.
The
guidelines make the following important point: ‘The MedDRA terminology is
multiaxial and more complex than common terminologies previously used.
Therefore, term selection should be reviewed by a qualified individual, a
person with medical back-ground and/or training and who is also trained in the
use of MedDRA’.
The reader of this chapter should refer to the guide-lines for examples and details. Accurate and consistent coding of data are vital for the appropriate analysis and evaluation of safety data.
Related Topics
