Volatile/Inhalation anaesthetics
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : General Anaesthetics
1. Ether (Diethyl ether) 2. Trichloro ethylene 3. Halothane 4. Methoxy Flurane 5. Enflurane 6. Isoflurane 7. Sevoflurane 8. Cyclopropane (Trimethylene) 9. Nitrous oxide (N2O) : SYNTHESIS AND DRUG PROFILE : Volatile/Inhalation anaesthetics
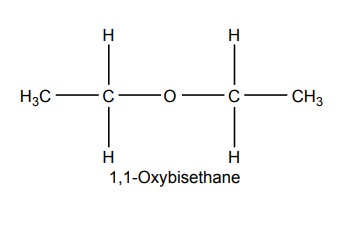
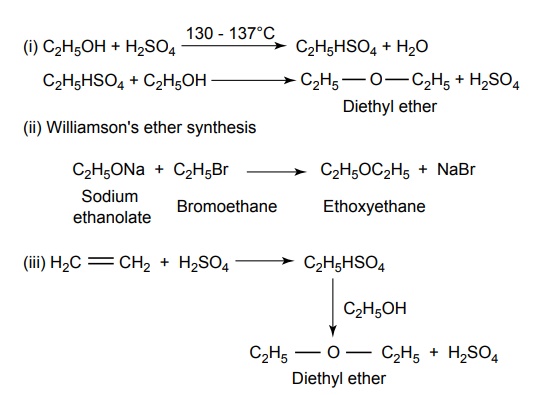
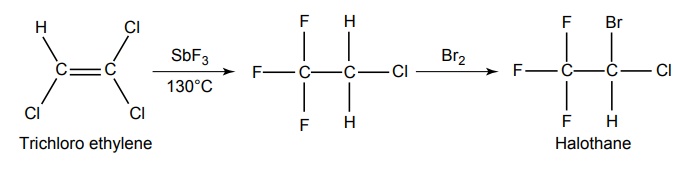
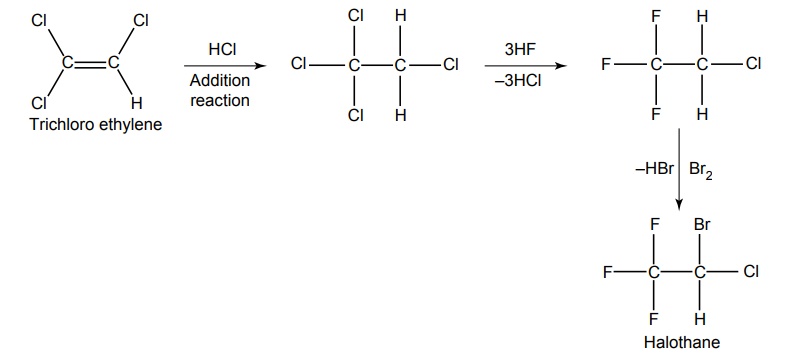
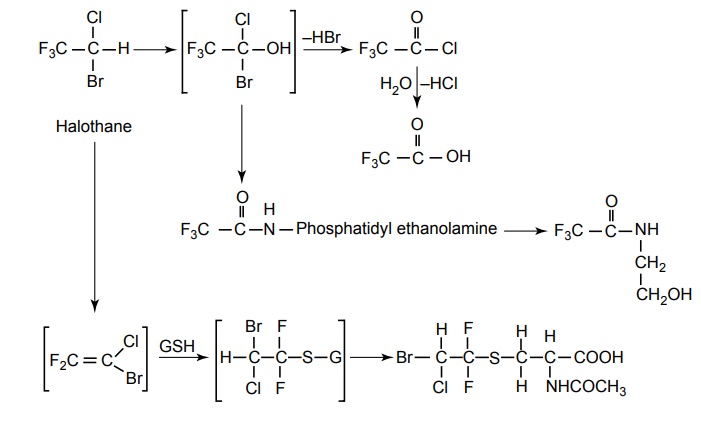
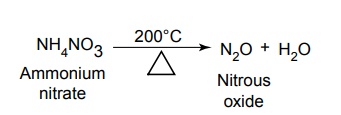
SYNTHESIS AND DRUG PROFILE Volatile/Inhalation anaesthetics Synthesis Properties and uses: It is a clear, colourless liquid, volatile, highly f lammable, soluble in water, miscible with alcohol, methylene chloride, and with fatty oils. Low molecular weight ethers display anaesthetic activity that increases along with toxicity as the chain length increases. Introduction of unsaturation into the aliphatic ether increases potency and also shortens induction and emergence. Ether is an absolute anaesthetic with pungent, irritant odour. It is f lammable and explosive at concentrations necessary for anaesthesia. Storage: It should be stored in well-closed airtight containers and protected from light, stored at a temperature of 8°C–15°C. Synthesis Properties and uses: It may be used sporadically as a weak volatile anaesthetic, administered through inhalation. It possess an excellent analgesic property. It is frequently employed in short surgical operations, where a mild anaesthesia having potent analgesia is desired. Synthesis Route I. From: Trichloro ethylene Route II. From: Trichloro ethylene Metabolism: It is metabolized to three major metabolic products, trifluroacetic acid, N-trifluro acetyl ethanolamine, and N-acetyl-s-(2-bromo,2 chloro-1,1-difluro ethyl)-1-cysteine Properties and uses: It is a clear, colourless, heavy, nonflammable liquid, slightly soluble in water, miscible with ethanol, and with trichloroethylene. Halothane lacks flammability. It may produce any depth of anaesthesia without causing hypoxia. Being a nonirritant, its inherent hypotensive effect retards capillary bleeding and renders a comparatively bloodless field. It is a potent, relatively safe general inhalation anaesthetic used in conjunction with N2O. For skeletal muscle relaxation, it is used with succinyl choline or tubocurarine. Storage: It should be stored in well-closed airtight containers, protected from light, at a temperature not exceeding 25°C in a nonreactive metal container. Synthesis Metabolism: It is metabolized in the liver to produce fluoride ions, oxalic acid, difluoro methoxyacetic acid, and dichloroacetic acid. The high concentration of fluoride ions causes renal damage. Properties and uses: It is a clear, colourless liquid, noninflammable and nonexplosive in air or oxygen in anaesthetic concentrations. It is the most potent of the inhalational agents. It is employed to cause light anaesthesia with deep analgesic and muscle relaxation feature, which makes it convenient for surgical operations. Synthesis Metabolism: The principal metabolites are difluromethoxy difluroacetic acid and fluoride ion. Properties and uses: It is a clear, colourless, volatile liquid with pleasant hydrocarbon-like odour. Soluble in water, miscible with organic solvents, chemically it is extremely stable. The induction of an emergence from anaesthesia and adjustment of anaesthetic depth during maintenance is smooth and moderately rapid. It is a noninflammable halogenated ether anaesthetic and provides rapid induction with no excitement. Synthesis Metabolism: It is metabolized to trifluroacetic acid and fluoride ion. Properties and uses: It is a clear, colourless, heavy liquid, insoluble in water, miscible with ethanol, and trichloroethylene. It resembles isomer enflurane in its properties. It is not flammable in air or oxygen. The depth of anaesthesia can be rapidly adjusted with it. Used for induction and maintenance of general anaesthesia. Storage: It should be stored in well-closed airtight containers and protected from light. Properties and uses: Low boiling liquid with a slight odour; miscible with most organic solvents including fats or oils; practically insoluble in water. It is a nonflammable, nonirritating agent. The physical properties of this compound result in a more rapid induction and termination of anaesthetic when observed with the currently used agents. Synthesis Properties and uses: It is nonirritant in nature and ensures rapid recovery from anaesthesia. The adverse effects are depressant effects on respiration, tendency to induce cardiac arrhythmias, and enhanced haemorrhage. Cyclopropane is an anaesthetic gas with a rapid onset of action. It may be used for analgesia, induction, or maintenance of anaesthesia. Properties and uses: It is a colourless gas, without appreciable odour to taste, soluble in water, freely soluble in alcohol, soluble in ether, or oils. This is the least toxic and least potent anaesthetic. It is a noninflammable, nonirritating, and a powerful analgesic agent. Nitrous oxide is a weak anaesthetic with good analgesic properties, and relatively no skeletal muscle relaxant properties. It is an inhalation anaesthetic of choice in dental surgery.1. Ether (Diethyl ether)
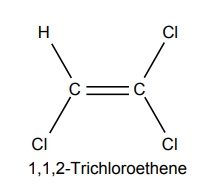
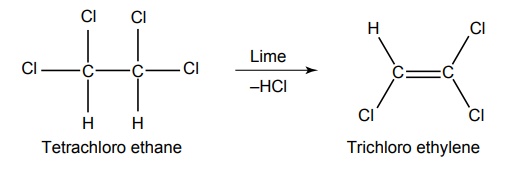
2. Trichloro ethylene
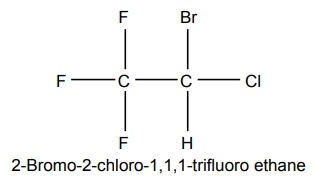
3. Halothane
4. Methoxy Flurane
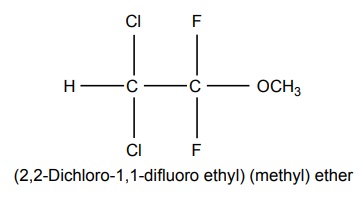
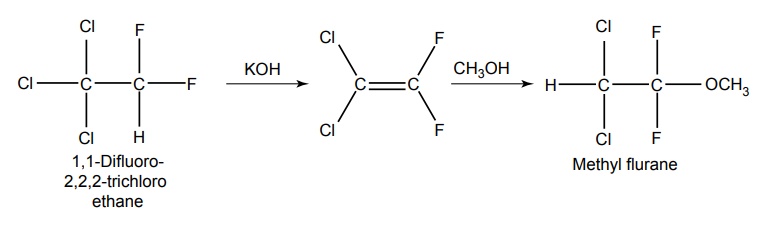
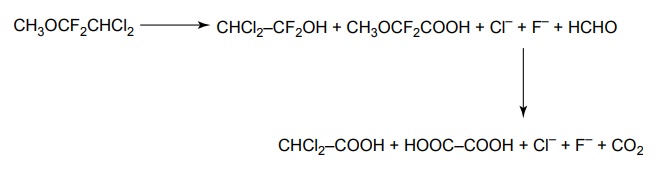
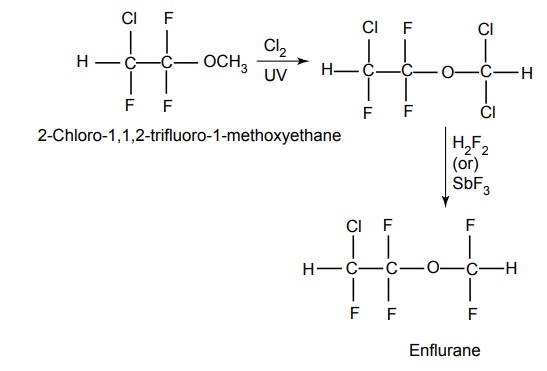
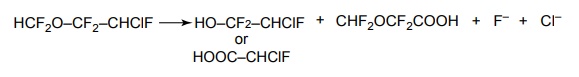
5. Enflurane
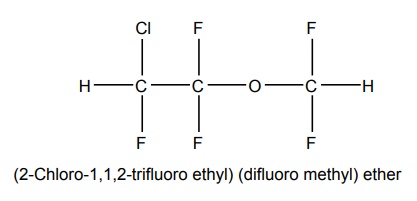
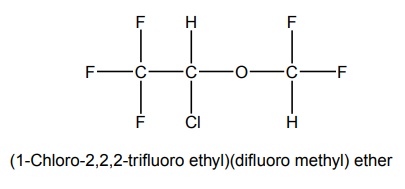
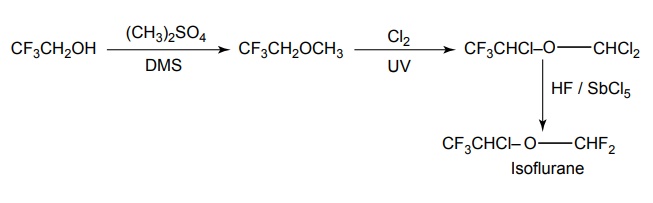
6. Isoflurane
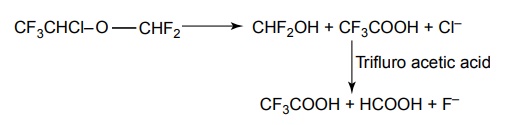
7. Sevoflurane
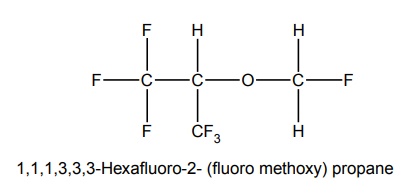
8. Cyclopropane (Trimethylene)
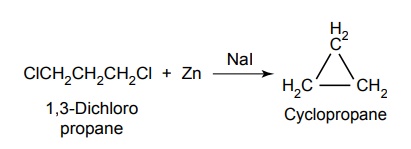
9. Nitrous oxide (N2O)
Related Topics
