Folate Antagonists
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Mechanisms of action of antibiotics and synthetic anti-infective agents
Folic acid is an important cofactor in all living cells. In the reduced form, tetrahydrofolate (THF), it functions as a carrier of single-carbon fragments, which are used in the synthesis of adenine, guanine, thymine and methionine.
FOLATE ANTAGONISTS
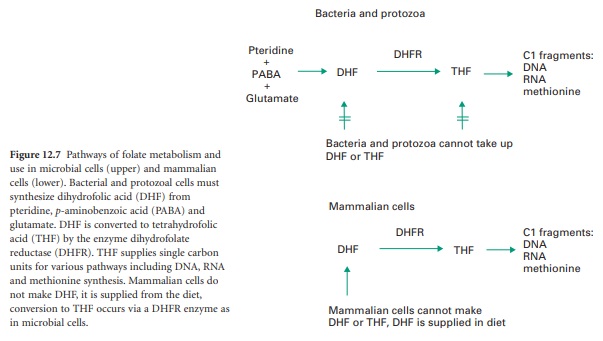
Folate Metabolism In Microbial And Mammalian Cells
Folic acid is an important cofactor
in all living cells. In the reduced form, tetrahydrofolate
(THF), it functions as a carrier of single-carbon fragments, which are used in the synthesis of adenine, guanine, thymine and methionine (Figure 12.7). One important folate-dependent enzyme is thymidylate synthetase, which produces TMP by transfer of the methyl
group from THF to UMP. In this and other
folate-dependent reactions THF is converted to dihydrofolic acid (DHF), which
must be reduced back to THF before it can participate again as a carbon
fragment carrier. The enzyme responsible for the reduction of DHF to THF is dihydrofolate reductase (DHFR) which uses the nucleotide NADPH2 as a cofactor. Bacteria, protozoa and mammalian cells all possess DHFR but there are sufficient differences in the
enzyme structure for inhibitors such as trimethoprim and pyrimethamine to inhibit the bacterial and protozoal enzymes
selectively without damaging the mammalian form. In the case of protozoa such as the Plasmodium species responsible for malaria, the DHFR is a double enzyme
which also contains the thymidylate synthetase activity.
There is another
fundamental difference between folate utilization in microbial and mammalian cells (Figure 12.7). Bacteria and protozoa are unable to take
up exogenous folate and must
synthesize it themselves. This is carried out
in a series of reactions involving first
the synthesis of dihydropteroic acid
from one molecule each of pteridine and p-aminobenzoic
acid (PABA). Glutamic acid is then
added to form DHF, which is reduced by DHFR
to THF. Mammalian cells
do not make their own DHF, instead they
take it up from dietary
nutrients and convert it to THF using DHFR.
Sulphonamides
Sulphonamides (e.g.
sulphamethoxazole and dapsone) are structural analogues of PABA (Figure
12.8). They competitively inhibit
the incorporation of PABA into dihydropteroic
acid and there
is some evidence
for their incorporation into
false folate analogues, which inhibit
subsequent metabolism. The presence of excess PABA will reverse
the inhibitory action
of sulphonamides, as will thymine, adenine,
guanine and methionine. However these nutrients are not normally
available at the site of infections for which
the sulphonamides are
used.
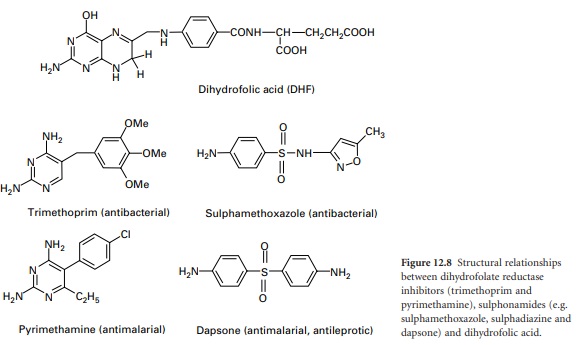
DHFR Inhibitors—Trimethoprim And Pyrimethamine
Trimethoprim is a selective inhibitor of bacterial DHFR. The bacterial enzyme is several
thousand times more sensitive than the mammalian enzyme. Pyrimethamine, likewise, is a selective inhibitor
of plasmodial DHFR. Both
are structural analogues of the dihydropteroic acid portion of the DHF substrate
(Figure 12.8). Crystal
structures of
the bacterial, plasmodial and mammalian DHFRs, each containing either bound substrate
or the inhibitors, have been
determined by X-ray diffraction studies. These show how inhibitors fit tightly into the
active site normally occupied
by the DHF substrate, forming a pattern
of strong hydrogen
bonds with amino acid residues and
water molecules lining
the site. Another DHFR is proguanil, a guanidine-containing
prodrug which is
metabolized in the liver to cycloguanil, an
active selective inhibitor of plasmodial DHFR.
Methotrexate is a potent DHFR
inhibitor that has
an analogous structure to the whole
DHF molecule, including the glutamate residue.
It has no selectivity towards microbial DHFR and therefore
cannot be used to treat infections; however, it is widely
used as an anticancer
agent. A derivative of methotrexate that is used for
treatment of Pneumocystis jirovecii infections in
AIDS patients is trimetrexate. Although it is very toxic
to mammalian cells, simultaneous administration of
leucovorin (formyl-THF or folinic acid) as an alternative source of folate which
cannot be taken
up by the organism protects host tissues. DHFR
inhibitors can be used in combination with a sulphonamide to achieve a
double interference
with folate metabolism. Suitable combinations with matching pharmacokinetic properties are sulphamethoxazole with
trimethoprim (the antibacterial co-trimoxazole) and dapsone with pyrimethamine (the antimalarial Maloprim).
Related Topics
