Resistance to β-lactam antibiotics
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Bacterial Resistance To Antibiotics
β-Lactam antibiotics act by inhibiting the carboxy/ transpeptidase or penicillin-binding proteins (PBPs) involved in the late stages of peptidoglycan biosynthesis.
RESISTANCE TO β-LACTAM ANTIBIOTICS
β-Lactam antibiotics act by inhibiting the carboxy/ transpeptidase or
penicillin-binding proteins (PBPs) involved in the late stages of peptidoglycan
biosynthesis. Although introduced nearly 60 years ago, β-lactam antibiotics
still represent the most widely used class of agents in the clinic today.
Resistance to many β-lactam agents is common and is most often caused by
β-lactamases or by mutation in the PBPs resulting in reduced affinity. Reduced
uptake and efflux are also seen, but they are less significant.
β-Lactamases
A number of different β-lactamases have
been described, but all share the feature of catalysing the ring-opening of the
β-lactam moiety (Figure 13.1).
Thus, the structural homology with the terminal D-Ala-d-Ala of maturing
peptidoglycan, shared by all β-lactam antibiotics, is lost.
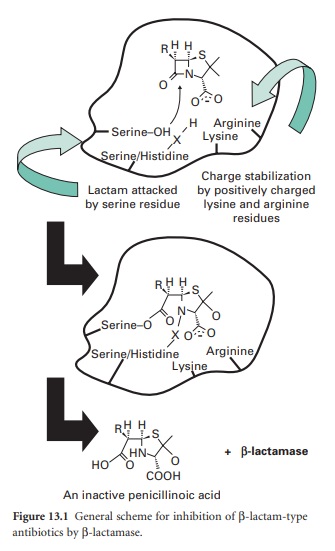
β-Lactamases may be chromosomal or
plasmid-borne, inducible or constitutive, and for this reason their terminology
can be confusing. A number of classification systems have been proposed,
including classes A-D based on peptide sequence. Classes A, C and D have a
serine at the active site, whereas class B enzymes have four zinc atoms at
their active site and these are also called metallo-β-lactamases. Class A
enzymes are highly active against benzylpenicillin; class B β-lactamases are
effective against penicillins and cephalosporins. Class C enzymes are usually
inducible, but mutation can lead to overexpression. Class D consists of the
OXA-type enzymes, which can hydrolyse oxacillin. Increasing resistance to
β-lactam agents, mainly by β-lactamase, prompted the discovery and introduction
of agents with greater β-lactam stability such as cephalosporins, carbapenems
and monobactams. Resistance first appeared in organisms such as Enterobacter cloacae and Pseudomonas aeruginosa, due to
mutations causing overproduction of the class C chromosomal AmpC β-lactamase.
Subsequently, in the late 1980s, resistance occurred in organisms such as Klebsiella pneumoniae and E. coli that lack an inducible AmpC enzyme.
Resistance was found to be mediated by plasmids encoding extended-spectrum
β-lactamases (ESBLs). These arose from mutational development of more
limitedspectrum β-lactamases such as TEM and SHV that either increased the size
of the active-site pocket or altered its binding characteristics to allow the
larger cephalosporins to enter and be broken down. TEM derivatives predominate,
possibly favoured by the use of ceftazidime and other slowly penetrating
cephalosporins. These mutations also increase the binding of clavulanic acid
and so these ESBLs remain susceptible to inhibition by this and other
β-lactamase inhibitors such as sulbactam and tazobactam, which are generally
ineffective against class C β-lactamases.
Continuing use of the third-generation
cephalosporins Serine-OH X Lysine Serine/Histidine Charge stabilization Lactam
attacked by positively charged by serine residue lysine and arginine residues
RH H S N O and the introduction of β-lactamase inhibitor combinations (clavulanate
with amoxycillin or ticarcillin, sulbactam with ampicillin, and tazobactam with
piperacillin; see section 4.2) resulted in the appearance of plasmids encoding
class C β-lactamases.After several unconfirmed reports, the first proof that a
class C β-lactamase had been captured on a plasmid came in 1990 when
transmis-sible resistance to α-methoxy and oxyimino-β-lactams was shown to be
mediated by an enzyme whose gene was 90% identical to the ampC gene of Ent. cloacae. They
have subsequently been found worldwide. Strains with Serine-O O X O
Serine/Histidine O plasmid-mediated AmpC enzymes are typically resistant
Arginine to aminopenicillins (ampicillin or amoxycillin), carboxLysine RH H S
HN + -lactamase HO O O COOH An inactive penicillinoic acid ypenicillins
(carbenicillin or ticarcillin) and ureidopenicillins (piperacillin). The
enzymes also provide resistance to the oxyimino cephalosporins (ceftazidime,
cefotaxime, ceftriaxone) and the 7-α-methoxy group (cefoxitin, cefmetazole and
moxalactam) as well as the monobactam aztreonam.
In December 2009, the first report of a
carbapenemase β-lactamase, referred to as New Delhi metallo-β-lactamase
(NDM-1), was recorded. It was discovered in a carbapenem-resistant K. pneumoniae strain isolated in Sweden from a
Swedish national who acquired the infection in India. The enzyme is one of the
class of B metallo-β-lactamases and is conferred by the gene blaNDM-1. This is
considered a serious threat to the carbapenem family of antibiotics.
β-Lactamase inhibitors
In addition to introducing agents with
increased stability to β-lactamase inhibition, β-lactamase inhibitors including
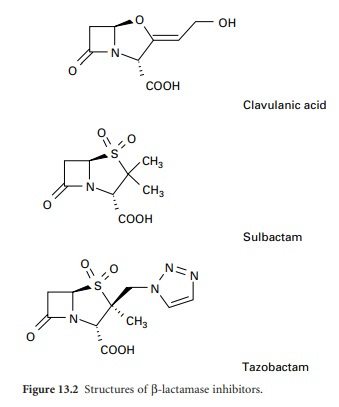
clavulanic acid, sulbactam and tazobactam have been developed (Figure 13.2).
Clavulanic acid is produced by a streptomyces and is a suicide inhibitor of
β-lactamases from a number of Gram-negative and Gram-positive organisms. These
β-lactamase inhibitors do not have any significant antimicrobial activity
against bacterial transpeptidases, but their combination with a β-lactam
antibiotic (see above) has extended the clinical usefulness of the latter.
Related Topics
