Resistance to Aminoglycoside Antibiotics
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Bacterial Resistance To Antibiotics
The aminoglycosides are hydrophilic sugars possessing a number of amino and hydroxy substituents. The amine groups are protonated at biological pH and it is the polycationic nature of the molecules that affords them their affinity for nucleic acids, particularly the acceptor (A) site of 16S ribosomal RNA.
RESISTANCE TO AMINOGLYCOSIDE ANTIBIOTICS
The aminoglycosides are hydrophilic
sugars possessing a number of amino and hydroxy substituents. The amine groups
are protonated at biological pH and it is the polycationic nature of the
molecules that affords them their affinity for nucleic acids, particularly the
acceptor (A) site of 16S ribosomal RNA. Aminoglycoside binding to the A site
interferes with the accurate recognition of cognate tRNA by rRNA during
translation and may also perturb translocation of the tRNA from the A site to
the peptidyl-tRNA site (P site). While high-level resistance in
aminoglycoside-producing microorganisms is by methylation of the rRNA, this is
not the mechanism of resistance in previously susceptible strains. The most common
mechanism for clinical aminoglycoside resistance is their structural
modification by enzymes expressed in resistant organisms, which compromises
their ability to interact with rRNA. There are three classes of these enzymes:
aminoglycoside phosphatases (APHs), aminoglycoside nucleotidyl-transferases
(ANTs) and aminoglycoside acetyltransferases (AACs). Within each class, there
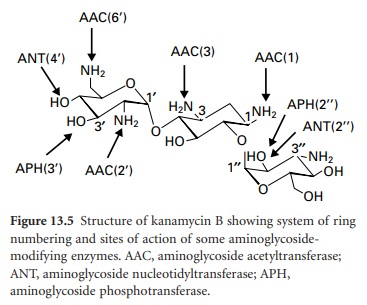
are enzymes with differing specificities around the sugars. There are four ANTs
(ANT(6), ANT(4′), ANT(3″) and ANT(2″)), seven APHs
(APH(3′), APH(2″), APH(3″), APH(6), APH(9), APH(4) and APH(7 ″)) and four AACs (AAC(2′), AAC(6′), AAC(1) and AAC(3)). There is also
a bifunctional enzyme, AAC(6′)-AAC(2″). Aminoglycosides are typically susceptible to
attack by multiple enzymes (Figure 13.5).
Attempts to circumvent these modifying enzymes have centred on structural
modifications. Examples include tobramycin which lacks the 3′-hydroxyl group and
is thus not a substrate for APH(3 ′), and
amikacin which has an acylated N-1 group and is not a substrate for several
modifying enzymes. Other strategies are exemplified by experimental compounds
such as 3′-oxo-kanamycin. This molecule is a substrate for APH(3′), but the phosphorylation product is unstable and
regenerates the original antibiotic.
Related Topics
