Barbiturates
| Home | | Pharmacology |Chapter: Essential pharmacology : Sedative -Hypnotics
Barbiturates have been popular hypnotics and sedatives of the last century upto 1960s, but are not used now to promote sleep or to calm patients. However, they are described first because they are the prototype of CNS depressants.
BARBITURATES
Barbiturates have been popular hypnotics and sedatives of the last century upto 1960s, but are not used now to promote sleep or to calm patients. However, they are described first because they are the prototype of CNS depressants.
Barbiturates are substituted derivatives of barbituric acid (malonyl urea). Barbituric acid as such is not a hypnotic but compounds with alkyl or aryl substitution on C5 are. Replacement of O with S at C2 yields thiobarbiturates which are more lipid-soluble and more potent. Barbiturates have variable lipid solubility, the more soluble ones are more potent and shorter acting. They are insoluble in water but their sodium salts dissolve yielding highly alkaline solution.
Pharmacological Actions
Barbiturates are general depressants for all excitable cells, the CNS is most sensitive where the effect is almost global, but certain areas are more susceptible.
CNS
Barbiturates produce dose-dependent effects:
sedation → sleep → anaesthesia → coma.
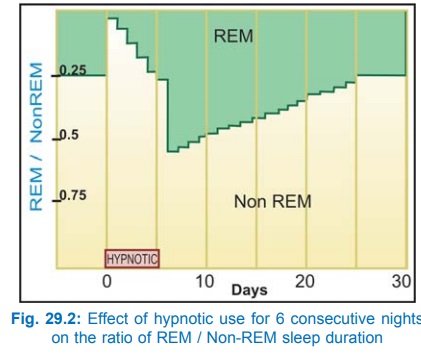
Hypnotic dose (100–200 mg of a short acting barbiturate) shortens the time taken to fall asleep and increases sleep duration. The sleep is arousable, but the subject may feel confused and unsteady if waken early. Night awakenings are reduced. REM and stage 3, 4 sleep are decreased; REM-NREM sleep cycle is disrupted. The effects on sleep become progressively less marked if the drug is taken every night consecutively. A rebound increase in REM sleep and nightmares is often noted when the drug is discontinued after a few days of use and it takes several days for normal pattern to be restored (Fig. 29.2). Hangover (dizziness, distortions of mood, irritability and lethargy) may occur in the morning after a nightly dose.
Sedative dose (smaller dose of a longer acting barbiturate) given at daytime can produce drowsiness, reduction in anxiety and excitability. However, barbiturates do not have selective anti-anxiety action. Barbiturates can impair learning, short-term memory and judgement. They have no analgesic action; small doses may even cause hyperalgesia. Euphoria may be experienced by addicts.
Barbiturates have anticonvulsant property. The 5phenyl substituted agents (phenobarbitone) have higher anti-convulsant : sedative ratio, i.e. they have specific anticonvulsant action independent of general CNS depression.
Higher dose of a barbiturate induces a predominance of slow, high voltage EEG activity. Progressive burst suppression occurs if dose is increased further. Barbiturates depress all areas of the CNS, but the reticular activating system is most sensitive; its depression is primarily responsible for inability to maintain wakefulness.
Mechanism Of Action
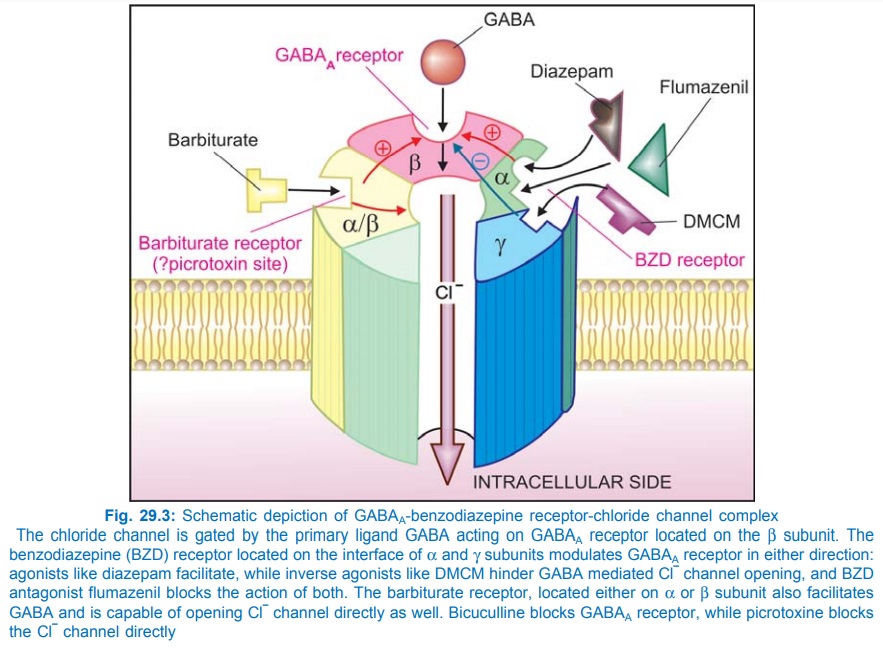
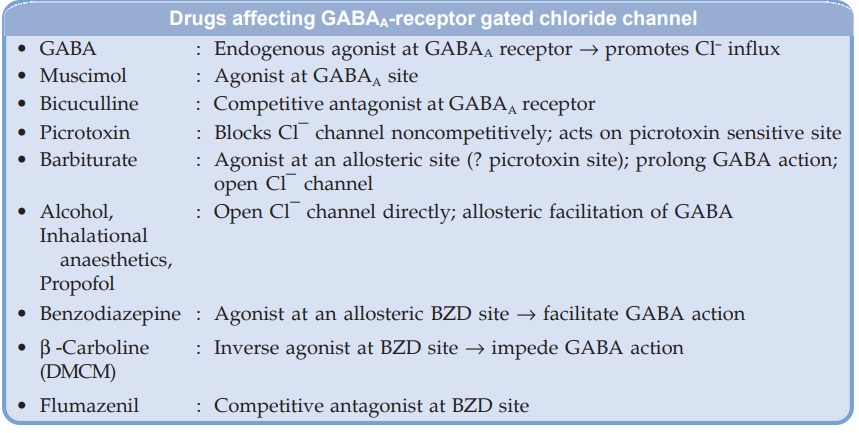
Barbiturates appear to act primarily at the GABA : BZD receptor–Cl¯ channel complex (see Fig. 29.3) and potentiate GABAergic inhibition by increasing the lifetime of Cl¯ channel opening induced by GABA. (contrast BZDs which enhance frequency of Cl¯ channel opening). They do not bind to the BZD receptor, but bind to another site (probably the picrotoxin sensitive site) on the same macromolecular complex to exert the GABAfacilitatory action. The barbiturate site appears to be located on α or β subunit, because presence of only these subunits is sufficient for their response. They also enhance BZD binding to its receptor. At high concentrations, barbiturates directly increase Cl¯ conductance (GABA mimetic action; contrast BZDs which have only GABAfacilitatory action) and inhibit Ca2+ dependent release of neurotransmitters. In addition they depress glutamate induced neuronal depolarization through AMPA receptors. At very high concentrations, barbiturates depress voltage sensitive Na+ and K+ channels as well. A dosedependent effect on multiple neuronal targets appears to confer the ability to produce any grade of CNS depression.
2. Other Systems
Respiration is depressed by relatively higher doses. Neurogenic, hypercapneic and hypoxic drives to respiratory centre are depressed in succession. Barbiturates donot have selective antitussive action.
CVS Hypnotic doses of barbiturates produce a slight decrease in BP and heart rate: magnitude of change not differing from that during normal sleep. Toxic doses produce marked fall in BP due to ganglionic blockade, vasomotor centre depression and direct decrease in cardiac contractility. Reflex tachycardia can occur, though pressor reflexes are depressed. However, the dose producing cardiac arrest is about 3 times larger than that causing respiratory failure.
Skeletal Muscle Hypnotic doses have little effect but anaesthetic doses reduce muscle contraction by depressing excitability of neuromuscular junction.
Smooth Muscles Tone and motility of bowel is decreased slightly by hypnotic doses; more profoundly during intoxication. Action on bronchial, ureteric, vesical and uterine muscles is not significant.
Kidney Barbiturates tend to reduce urine flow by decreasing BP and increasing ADH release. Oliguria attends barbiturate intoxication.
Pharmacokinetics
Barbiturates are well absorbed from the g.i. tract. They are widely distributed in the body. The rate of entry into CNS is dependent on lipid solubility. Highlylipid soluble thiopentone has practically instantaneous entry, while less lipidsoluble ones (pentobarbitone) take longer; phenobarbitone enters very slowly. Plasma protein binding varies with the compound, e.g. thiopentone 75%, pentobarbitone 35%, phenobarbitone 20%. Barbiturates cross placenta and are secreted in milk; can produce effects on the foetus and suckling infant.
Three processes are involved in termination of action of barbiturates: the relative importance of each varies with the compound.
Redistribution It is important in the case of highly lipidsoluble thiopentone and others. After their i.v. injection, consciousness is regained in 6–10 min due to redistribution (see Ch. No. 2) while the ultimate disposal occurs by metabolism (t½ of elimination phase is 9 hours). Effect of single dose of short acting barbiturate may last just 6–10 hours due to redistribution, while elimination t½ is 12–40 hours.
Metabolism Drugs with intermediate lipidsolubility (shortacting barbiturates) are primarily metabolized in liver by oxidation, dealkylation and conjugation. Their plasma t½ ranges from 12–40 hours.
Excretion Barbiturates with low lipidsolubility (longacting agents) are significantly excreted unchanged in urine. The t½ of phenobarbitone is 80–120 hours. Alkalinization of urine increases ionization and excretion. This is most significant in the case of longacting agents.
Barbiturates induce hepatic microsomal enzymes and increase the rate of their own metabolism as well as that of many other drugs.
Uses
Except for phenobarbitone in epilepsy (Ch. No. 30) and thiopentone in anaesthesia (Ch. No. 27), barbiturates are seldom used now. As hypnotic and anxiolytic they have been superseded by BZDs. They are occasionally employed as adjuvants in psychosomatic disorders. The enzyme inducing property of phenobarbitone can be utilized to hasten clearance of congenital non-haemolytic jaundice and kernicterus.
Phenobarbitone 30–60 mg oral OD–TDS; 100–200 mg i.m./i.v.
GARDENAL 30, 60 mg tab, 20 mg/5 ml syr; LUMINAL 30 mg tab; PHENOBARBITONE SOD 200 mg /ml inj
Adverse Effects
Side Effects
Hangover was common after the use of barbiturates as hypnotic. On repeated use they accumulate in the body—produce tolerance and dependence. Mental confusion, impaired performance and traffic accidents may occur (also see Ch. No. 30).
Idiosyncrasy
In an occasional patient barbiturates produce excitement. This is more common in the elderly. Precipitation of porphyria in susceptible individuals.
Hypersensitivity
Rashes, swelling of eyelids, lips, etc.— more common in atopic individuals.
Tolerance and Dependence
Both cellular and pharmacokinetic (due to enzyme induction) tolerance develops on repeated use. However, fatal dose is not markedly increased: addicts may present with acute barbiturate intoxication. There is partial cross tolerance with other CNS depressants.
Psychological as well as physical dependence occurs and barbiturates have considerable abuse liability—one of their major disadvantages. Withdrawal symptoms are—excitement, hallucinations, delirium, convulsions; deaths have occurred.
Acute Barbiturate Poisoning
Mostly suicidal, sometimes accidental; infrequently encountered now due to in-availability of barbiturates. However, the principles of treatment apply to any CNS depressant poisoning.
Manifestations are due to excessive CNS depression—patient is flabby and comatose with shallow and failing respiration, fall in BP and cardiovascular collapse, renal shut down, pulmonary complications, bullous eruptions.
Lethal dose depends on lipid solubility. It is 2–3 g for the more lipid-soluble agents (short-acting barbiturates) and 5–10 g for less lipid-soluble phenobarbitone.
Treatment
1. Gastric lavage; leave a suspension of activated charcoal in the stomach to prevent absorption of the drug from intestines.
2. Supportive measures: such as, patent airway, assisted respiration, oxygen, maintenance of blood volume by fluid infusion and use of vasopressors— dopamine may be preferred for its renal vasodilating action.
3. Alkaline diuresis: with sodium bicarbonate 1 meq/ kg i.v. with or without mannitol is helpful only in the case of longacting barbiturates which are eliminated primarily by renal excretion.
4. Haemodialysis and Haemoperfusion (through a column of activated charcoal or other adsorbants) is highly effective in removing longacting as well as shortacting barbiturates.
There is no specific antidote for barbiturates. In the past, analeptics like metrazol, bemegride, etc. have been used in an attempt to awaken the patient. This is dangerous, may precipitate convulsions while the patient is still comatose—mortality is increased. The emphasis now is on keeping the patient alive till the poison has been eliminated.
Contraindications
1. Acute intermittent porphyria—barbiturates exacerbate it by inducing microsomal enzymes (δ aminolevulinic acid synthetase) and increasing porphyrin synthesis.
2. Liver and kidney disease.
3. Severe pulmonary insufficiency, e.g. emphysema.
4. Obstructive sleep apnoea.
Interactions
· Barbiturates induce the metabolism of many drugs and reduce their effectiveness—warfarin, steroids (including contraceptives), tolbutamide, griseofulvin, chloramphenicol, theophylline.
· Additive action with other CNS depressants —alcohol, antihistamines, opioids, etc.
· Sodium valproate increases plasma concentration of phenobarbitone.
· Phenobarbitone competitively inhibits as well as induces phenytoin and imipramine metabolism: complex interaction.
· Phenobarbitone decreases absorption of griseofulvin from the g.i.t.
