Biosynthesis
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Prostaglandins
PGs are found in virtually all the tissues and organs. They are autocrine and paracrine lipid mediators that act on platelet endothelium, uterine tissues, and mast cells among others.
BIOSYNTHESIS
PGs are
found in virtually all the tissues and organs. They are autocrine and paracrine
lipid mediators that act on platelet endothelium, uterine tissues, and mast
cells among others. The biosynthesis of PGE and PGF has been thoroughly
established and both of them are derived from arachidonic acid. Two types of
pathways have been proposed and are designated as follows:
2.
Lipoxygenase
pathway
CYCLOOXYGENASE PATHWAY
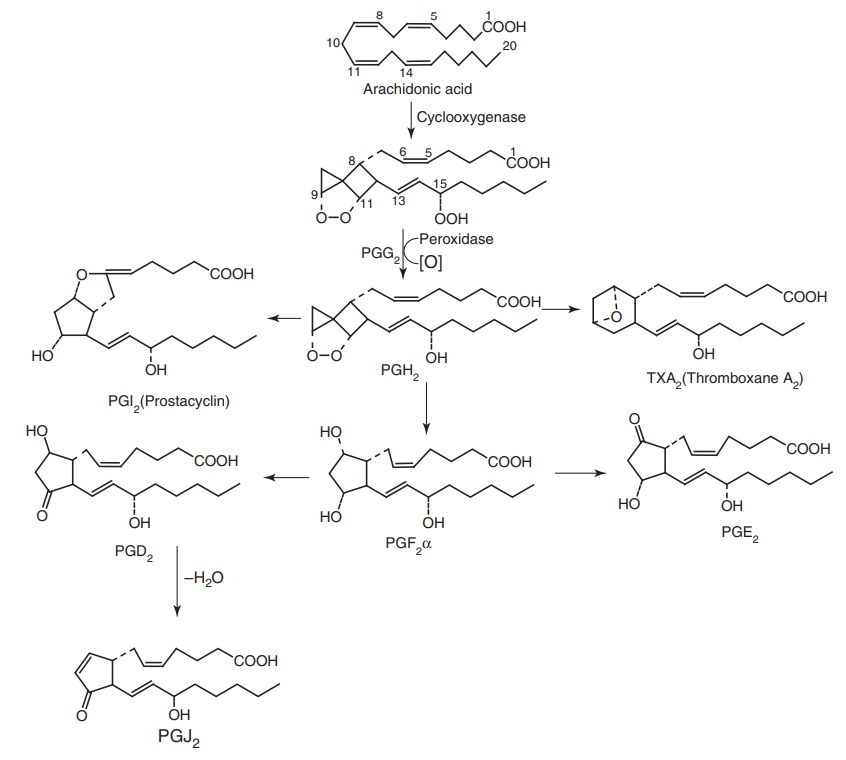
Arachidonic
acid is derived from dietary linoleic acid. It is present as a conjugated
component of the phospholipid matrix of the most cellular membrane. Release of
free arachidonic acid is due to the stimulation of phospholipase enzyme in
response to some traumatic events (tissues damage, toxin, exposure, and
hormonal stimulation). The first step in this pathway is the interaction of arachidonic
acid with PGH synthase, a haemoprotein, that catalyses both the addition of
oxygen and subsequent reduction (peroxide activity) of the 15th position of
hydroperoxide to 15(s) configuration alcohol prostaglandin H2 (PGH2).
PGH synthase is also called as cyclooxygenase I (COX-1) or cyclooxygenase II
(COX-2). NSAIDs inhibit PGs synthesis; leading to relief of the pain, fever,
and inflammation.
PGH2
serves as a substrate for specific enzymes, leading to the production of various
PGs, TXA2, and PGI2. While PGE2 is formed by
the action of endoperoxide isomerase on PGH2 and PGD2 by
the action of isomerase or glutathione-s-transferase on PGH2. PGF2
is formed from PGH2 via endoperoxidase reductase. Thromboxane
synthetase acts on PGH2 to produce thromboxane A2.
LIPOXYGENASE PATHWAY
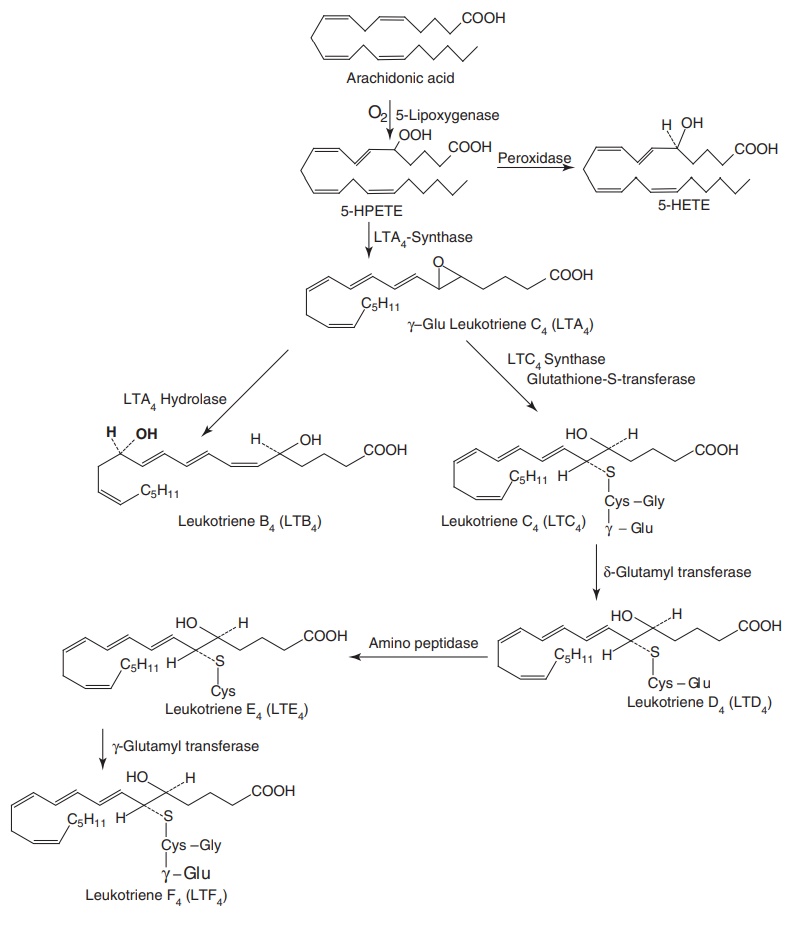
Lipoxygenase
are a group of enzymes that oxidize polyunsaturated fatty acid possessing two cis double bond separated by a methylene
group to produce lipid peroxides. Arachidonic acid is metabolized to form a
number of hydroperoxy eicosatetraenoic acid (HPETE) derivatives. These enzymes
differ in the position at which they peroxidize
arachidonic acid and in the tissues specificity. For example, platelets possess
only 12-lipoxygenase, whereas leukocytes possess both 12-lipoxygenase and
5-lipoxygenase. Leukotriens are products of the 5-lipoxygenase pathways and are
divided into major classes.
Hydroxylate
eicosotetraenoic acid (LTs) is represented by lymphotoxin β4 (LTB4)
and peptido leukotrienes (PLTs), such as leukotriene C4 (LTC4),
leukotriene D4 (LTD4), and LTE4. Lipoxygenase produces
leukotrienase from 5-HPETE. Lysine epsilon-aminotransferase (LAT) synthetase
converts 5-HPETE to unstable epoxide termed leukotriene A4 (LTA4)
that may be converted by the enzymes into the leukotriene, LTB4 or
by LTC4 to other leukotrienes (e.g. LTD4, LTE4,
and LTF4), and reconjugation with glycine and glutamic acid,
respectively.
SAR of PGs

In the upper chain: Methyl esters (misoprostol), sulphonamide
(sulprostone), and hydroxyl group (rioprost) possess greater activity than
natural PGs.
In the cyclopentane ring: Variation in the cyclopentane ring results in
a reduction in the PG activity. Enlargement of the ring or reduction of the
ring leads to inactive compounds. Replacement of the carbon atom of
cyclopentane ring by O, S, and N also leads to inactive compounds. Replacement
of 9-keto group with = CH2 group gives active (metenprost) PG.
In the lower chain: C-15 hydroxyl group is protected (from
metabolism) by the introduction of methyl group at C-15 and gem dimethyl group
at C-16. The shifting of C-15 hydroxyl to C-16 position increases the metabolic
stability of alkoxy, phenoxy (enprostil, sulprostone) analogues, and they are
more active than natural PGs. Introduction of acetylinic group at C-13 and C-14
increase the leuteolytic activity.
Related Topics
