p-Amino phenol derivatives
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Analgesics, Antipyretics, and NSAIDs
i. Phenacetin (Acetophenetidin) ii. Paracetamol (Metacin, Tylenol, Tapar, Calpol) - SAR of p-amino Phenol Derivatives, Synthesis and Drug Profile
p-Amino phenol
derivatives
These
derivatives possess analgesic and antipyretic action, but lack anti-inflammatory
effects. Acetanilide was introduced into the therapy in 1886 as an
antipyretic–analgesic agent. However, it was subsequently found to be too
toxic, having been associated with methemaglobinemia and jaundice.
Phenacetin
was introduced in the following year and was widely used but was withdrawn
recently because of its nephrotoxicity. Acetaminophen (paracetamol) was
introduced in 1893 and it remains the only useful agent of this group used as
an antipyretic and an analgesic agent.
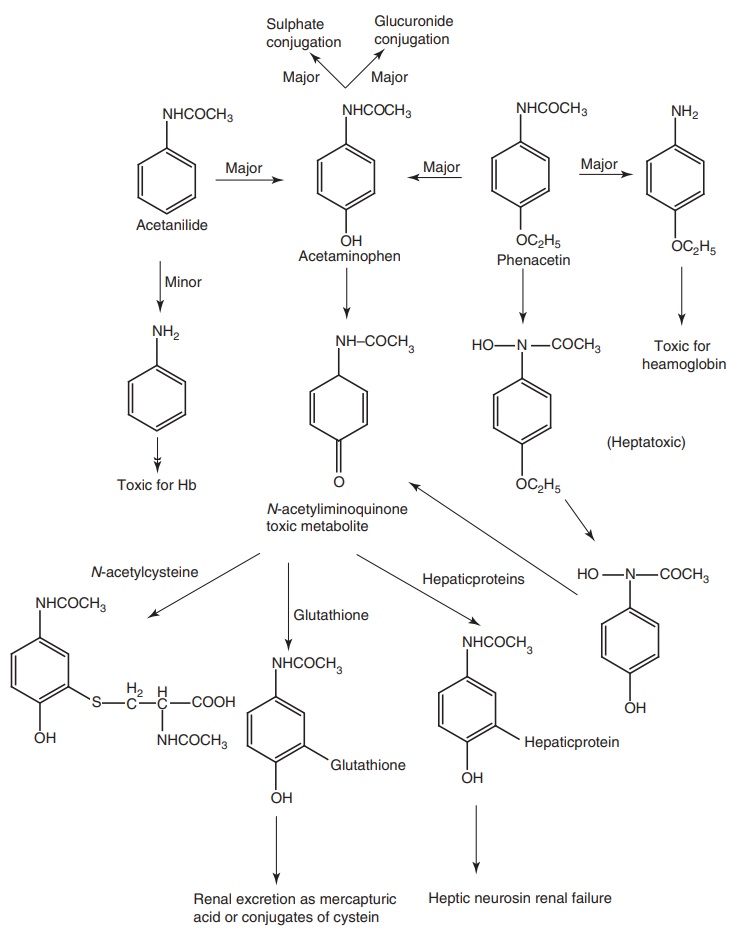
Metabolism of para aminophenol derivatives: These drugs undergo hydrolysis to yield
aniline derivatives that produce directly or through their conversion to
hydroxylamine derivatives, such as Acetaminophen that undergoes rapid first pass
metabolism in the GIT to o-sulphate
conjugate. The N-hydroxylamine is then
converted into a reactive toxic metabolite, acetiminoquinone, which produce
toxicity to the kidney and liver in conjugation with hepatic glutathione to
form mercapturic acid or cysteine conjugates.
SAR of p-amino Phenol Derivatives
1.
Etherification
of the phenolic function with methyl or propyl groups produces derivatives with
greater side effects than ethyl derivatives.
2.
Substituents
of the nitrogen atom, which reduce the basicity, also reduce activity unless
the substituent is metabolically labile. Example - acetyl groups.
3.
Amides
derived from aromatic acid. Example - N-phenyl
benzamides that are less active or inactive.
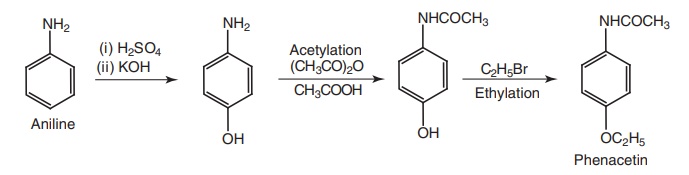
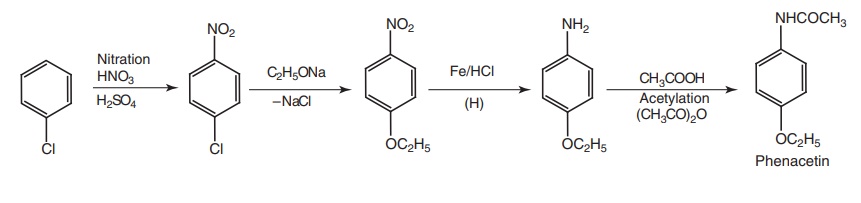
i. Phenacetin (Acetophenetidin)
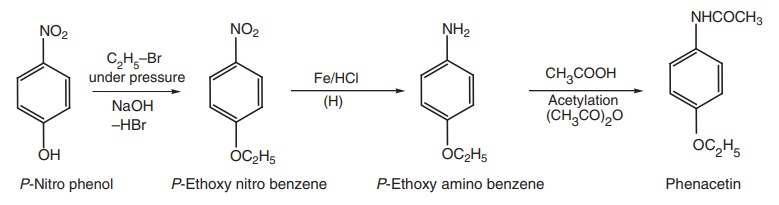
Synthesis
Route I. From: p-nitro phenol
Route II. From: aniline
Route III. From: chlorobenzene
Properties and uses: It exists as a white glistering powder with a
bitter taste, sparingly soluble in water and soluble in chloroform. It is an
analgesic and an antipyretic with similar effectiveness as an aspirin. It has a
greater potential for toxicity (hemolytic anaemia and methemoglobinaemia) than
paracetamol.
Dose: Usual dose as oral for adults is 300 mg to 2 g per day.
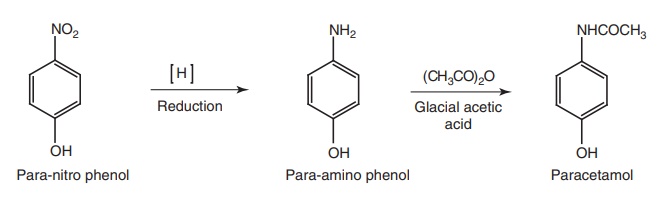
ii. Paracetamol (Metacin, Tylenol, Tapar, Calpol)
Synthesis
Properties and uses: Paracetamols exist as white crystalline powder,
sparingly soluble in water, soluble in alcohol, and very slightly soluble in
methylene chloride. Paracetamols produce antipyresis by acting on the
hypothalamic heat-regulating centre and analgesia by elevating the pain
threshold. Hepatic necrosis and death have been observed following over dosage;
hepatic damage is likely in an adult who takes more than 10 g in a single dose
or if a 2-year old child takes more than 3 g.
Assay: Dissolve the sample in a mixture of water and dilute sulphuric
acid (1:3), reflux, cool, and dilute with water. Add dilute hydrochloric acid
and titrate against 0.1 M cerium sulphate using ferroin as an indicator until a
greenish-yellow colour is obtained. Perform a blank titration.
Dose: Usual oral adult dose is 500 mg to 1 g for three or four times a
day.
Dosage forms: Paracetamol tablets I.P, B.P., Paracetamol syrup I.P.,
Co-codamol tablets B.P., Effervescent Co-codamol tablets B.P., Co-dydramol
tablets B.P., Co-proxamol tablets B.P., Paracetamol capsules B.P., Paediatric
paracetamol oral solution B.P., Paracetamol oral suspension B.P., Paracetamol
suppositories B.P., Dispersible paracetamol tablets B.P., soluble paracetamol
tablets B.P.
Related Topics
