Salicylates
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Analgesics, Antipyretics, and NSAIDs
i. Aspirin (Emipirin, Bufferin) ii. Sodium salicylate iii. Salsalate (Disalacid, Saloxium) iv. Sulphasalazine (Azultidine, Azaline) v. Diflunisal
Salicylates
Salicylates
not only posses antipyretic, analgesic, and anti-inflammatory properties, but
also other actions that have been proven to be therapeutically beneficial
because salicylates promote the excretion of uric acid and they are useful in
the treatment of gouty arthritis. More attention has been given to the ability
of salicylates (aspirin) to inhibit platelet aggregation, which may contribute
to heart attack and strokes, and hence, aspirin reduces the risk of myocardial
infarction. In addition, a recent study suggested that aspirin and other NSAIDs
might be protective against colon cancer.
Structural Activity Relationship (SAR) of Salicylates
·The active moiety of salicylates is salicylate
anion, side effects of aspirin, particularly GIT effects appear to be
associated with the carboxylic acid functional group.
·Reducing the acidity of the carboxy group
results in a change in the potency of activity. Example—
the
corresponding amide (salicylamide) retain the analgesic action of salicylic
acid, but is devoid of anti-inflammatory properties.
·Substitution on either the carboxyl or phenolic
hydroxyl group may affect the potency and toxicity.
Benzoic acid
itself has only week activity.
·Placement of the phenolic hydroxyl group at meta
or para to the carboxyl group abolish the activity.
·Substitution of halogen atom on the aromatic
ring enhances potency and toxicity.
·Substitution of aromatic ring at the 5th
position of salicylic acid increase anti-inflammatory activity (diflunisal).
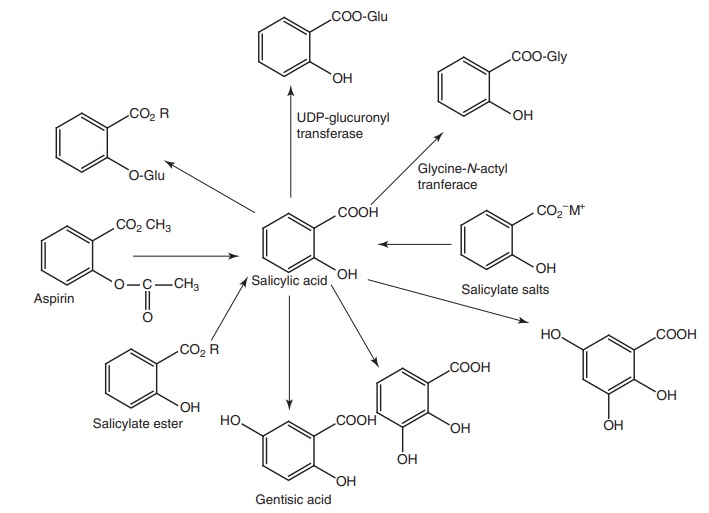
Metabolism of salicylic acid derivatives: The initial route of metabolism of these
derivatives is their conversion to salicylic acid, which is excreted in urine
as free acid (10%) or undergoes conjugation with either glycine to produce the
major metabolites of salicylic acid (75%) or with glucuronic acid to form
glucuronide (15%). In addition, small amount of metabolites resulting from
microsomal aromatic hydroxylation leads to gentisic acid.
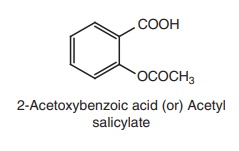
i. Aspirin (Emipirin, Bufferin)
Synthesis
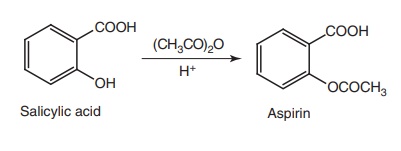
Properties and uses: Aspirin is a white crystalline powder, slightly
soluble in water and soluble in alcohol, indicated for the relief of minor
aches and mild-to-moderate pain in the conditions such as arthritis and related
arthritic condition. Also used in myocardial infarction prophylaxis.
Assay: Dissolve the sample in alcohol and add 0.5 M sodium hydroxide.
Allow to stand and titrate against 0.5 M hydrochloric acid using phenolphthalein as an
indicator. Perform a blank titration.
Dose: Usual adult dose: 300 to 650 mg every 3 or 4 h orally or 650 mg
to 1.3 g as the sustained-release tablet every 8 h; rectal, 200 mg to 1.3 g
three or four times a day.
Dosage forms: Aspirin tablets I.P., B.P., Dispersible aspirin tablets B.P.,
Effervescent soluble aspirin tablets B.P., Gastro-resistant aspirin tablets
B.P., Aspirin and Caffeine tablets B.P., Co-codaprin tablets B.P., Dispersible
co-codaprin tablets B.P.
ii. Sodium salicylate
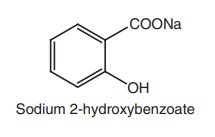
Synthesis
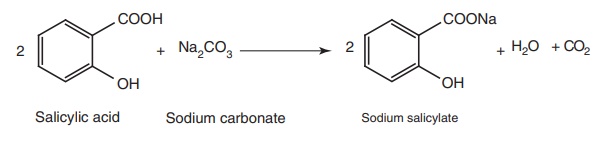
Properties and uses: Sodium salicylate is a white crystalline powder,
soluble in water, sparingly soluble in alcohol. It is used for fever and for
the relief of pain. It also possesses anti-inflammatory actions similar to
aspirin and symptomatic therapy of gout.
Assay: Dissolve the sample in anhydrous acetic acid and titrate against
0.1 M perchloric acid. Determine the end-point potentiometrically.
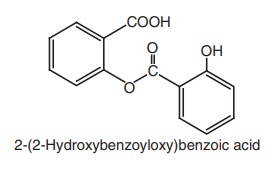
iii. Salsalate (Disalacid, Saloxium)
Synthesis
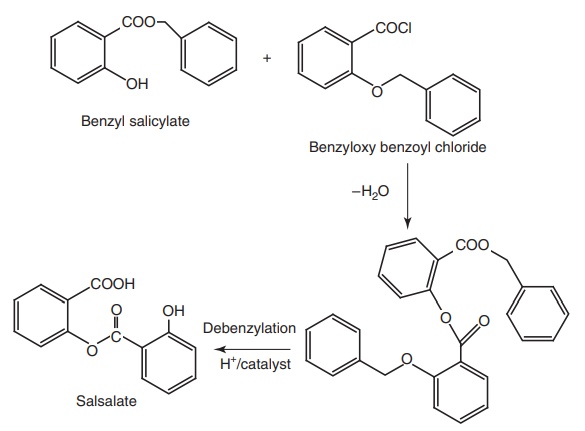
Properties and uses: Salsalate or salicylsalicylic acid is a dimer of
salicylic acid. It is insoluble in gastric juice, but is soluble in the small
intestine where it is partially hydrolyzed into two molecules of salicylic acid
and absorbed. It does not cause GI blood loss. It has antipyretic, analgesic,
and anti-inflammatory properties similar to those of aspirin. It is employed in
the treatment of rheumatoid arthritis and other rheumatic disorders.
Dose: Usual adult dose is 325–1000 mg 2–3 times a day, orally.
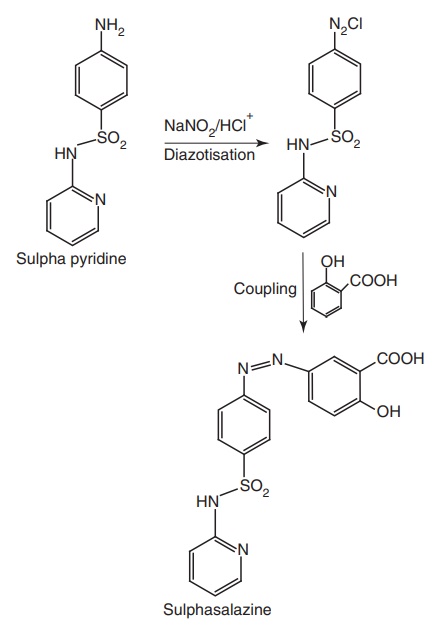
iv. Sulphasalazine (Azultidine, Azaline)
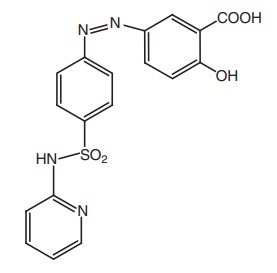
Properties and uses: Sulphasalazine is a bright yellow or
brownish-yellow fine powder, practically insoluble in water and methylene
chloride, very slightly soluble in alcohol, soluble in dilute solutions of
alkali hydroxides. Sulphasalazine is a mutual prodrug. In large intestine, it
is activated to liberate 5-amino salicylic acid, which in turn inhibits PG
synthesis and the sulphapyridine is useful for the treatment of infection. Hence,
sulphasalazine is used in the treatment of ulcerative colitis.
Synthesis
Assay: Dissolve and dilute the sample in 0.1 M sodium hydroxide and add
0.1 M acetic acid and measure the absorbance at the maxima of 359 nm using
ultraviolet spectrophotometer. Prepare a standard solution at the same time and
in the same manner, using sulphasalazine reference standard.
Dose: Dose orally is initially 3–4 g daily, followed by 500 mg four
times a day for maintenance.
Dosage forms: Sulphasalazine tablets B.P.
v. Diflunisal
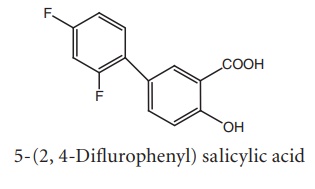
Synthesis
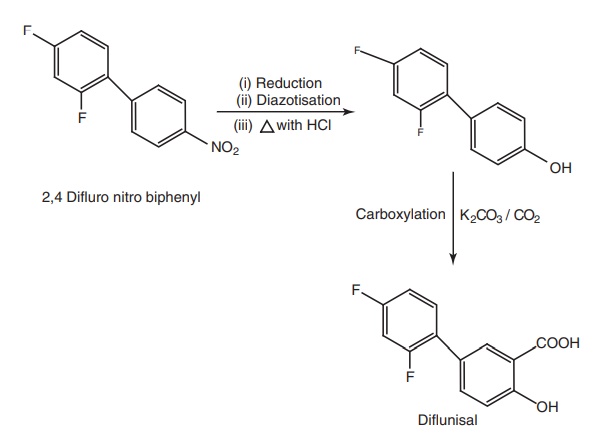
Properties and uses: Diflunisal is a white crystalline powder,
practically insoluble in water, soluble in alcohol, and dilute solutions of
alkali hydroxides. It is more potent than aspirin, but produces fewer side
effects, and has a biological half-life 3–4 times greater than that of aspirin.
It is a nonselective cyclooxygenase inhibitor used as antipyretic, analgesic,
and anti-inflammatory.
Assay: Dissolve the sample in methanol, add water, and titrate
against 0.1 M sodium hydroxide using phenol red as indicator, until the colour
changes from yellow to reddish-violet.
Related Topics
