Arylalkanoic acids
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Analgesics, Antipyretics, and NSAIDs
a. Indole acetic acid derivatives i. Indomethacin (Indocin, Indocid) b. Indeneacetic acid derivatives i. Sulindac (Clinoril) c. Pyrrole acetic acid derivative i. Tolmetin Sodium (Tolectin) ii. Zomepirac (Zomax) d. Aryl and heteroaryl acetic/propionic acid derivatives i. Ibuprofen (Brufen, Motrin) ii. Ibufenac iii. Diclofenac (Voltaren, Voveran) iv. Naproxen (Naprosyn) v. Fenoprofen (Nalton) vi. Ketoprofen (Orudis) vii. Ketoprofen viii. Flurbiprofen (Ansaid) viii. Caprofen ix. Ketorolac (Acular, Ketodrops, Ketlur) x. Etodolac
Arylalkanoic acids
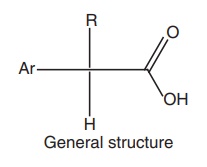
SAR of Arylalkanoic Acids
1.
The centre
of acidity is usually located one carbon atom adjacent to a flat surface
represented by an aromatic or hetero aromatic ring.
2.
The distance
between these centres is critical because increasing this distance to two or
three carbons generally decreases activity.
3.
All agents
possess a centre of acidity, which can be represented by a carboxylic acid and
hydroxamic acid, a sulphonamide or a terazole.
4.
Substitution
of a methyl group on the carbon atom separating the aromatic ring leads to enhancement
of anti-inflammatory activity.
a. Indole acetic acid derivatives
i. Indomethacin (Indocin, Indocid)
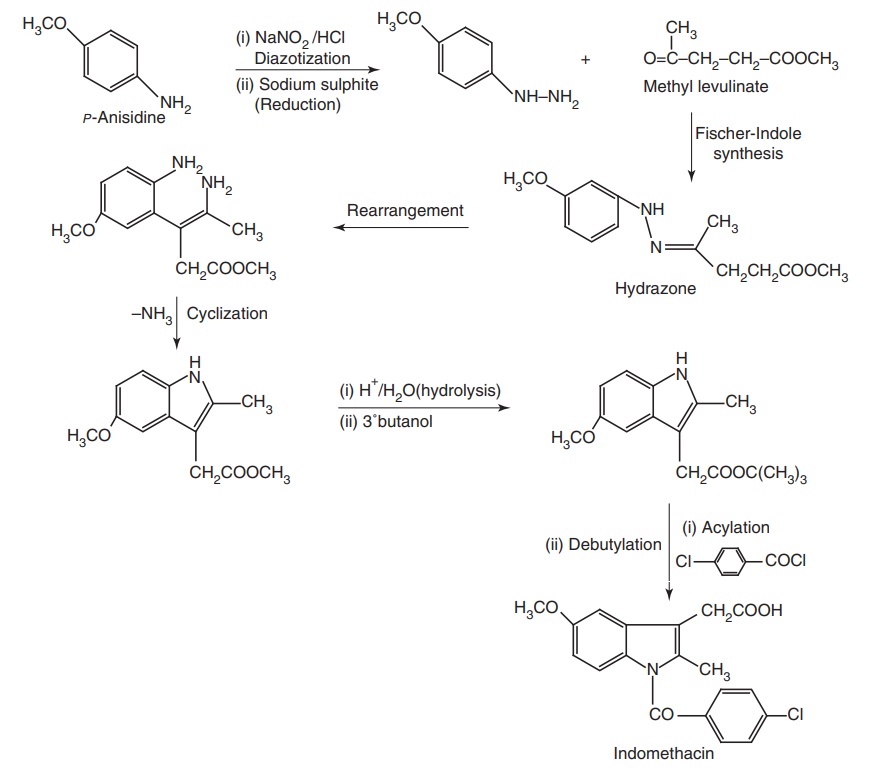
Synthesis
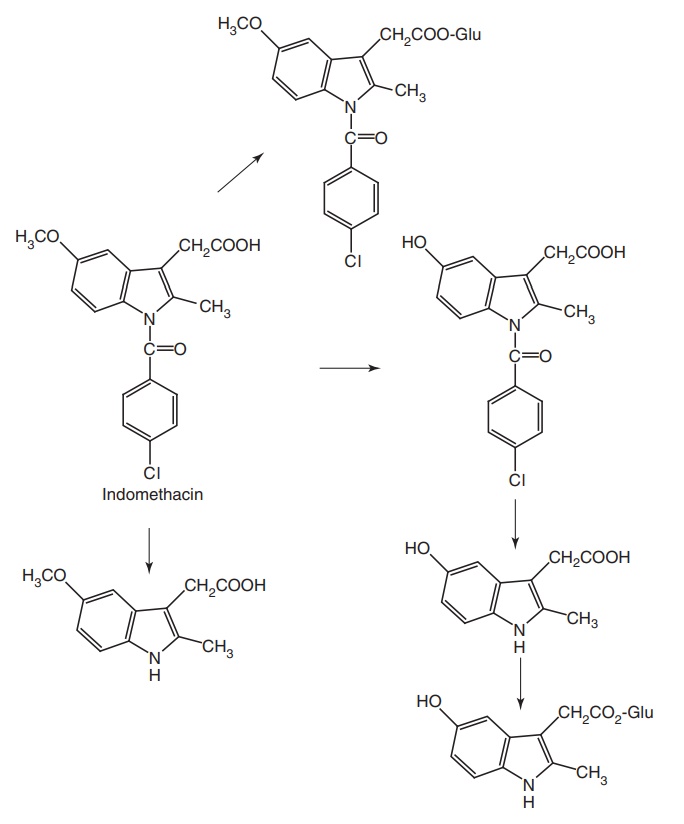
Metabolism: It is converted into inactive metabolites, that is, 50% of single
dose is 5-O-demethylated and 10%
conjugated with glucuronic acid. Nonhepatic enzymes hydrolyze indomethacin to N-deacetylated metabolite.
Properties and uses: It is a white or yellow crystalline powder,
insoluble in water and sparingly soluble in alcohol. Indomethacin is more
effective than aspirin. The most frequent side effects are gastric distress and
headache. It also has been associated with peptic ulceration, blood disorders,
and possible death (these side effects appear to be closely related and
sometimes can be minimized by reducing the dose). It is not recommended for use
in children because of possible interference with the resistance to infection.
Used as anti-inflammatory and analgesic in rheumatic arthritis, spondylitis, and
to lesser extent in gout.
Assay: Dissolve the sample in acetone and pass nitrogen for 15 min and
titrate with 0.1 M sodium hydroxide using phenolphthalein as indicator.
Dose: In gout, usual adult dose orally is 100 mg initially, followed
by 50 mg three times a day until pain is relieved. As an antirheumatic by oral
route, the dose is 50 mg two or three times a day. And as an antipyretic, the
dose is orally 25–50 mg three times a day.
Dosage forms: Indometacin capsules I.P., B.P., Indometacin Suppositories I.P.,
B.P.
b. Indeneacetic acid derivatives
i. Sulindac (Clinoril)
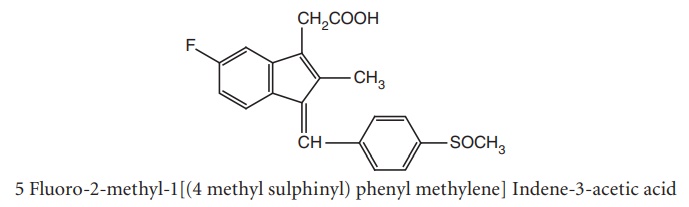
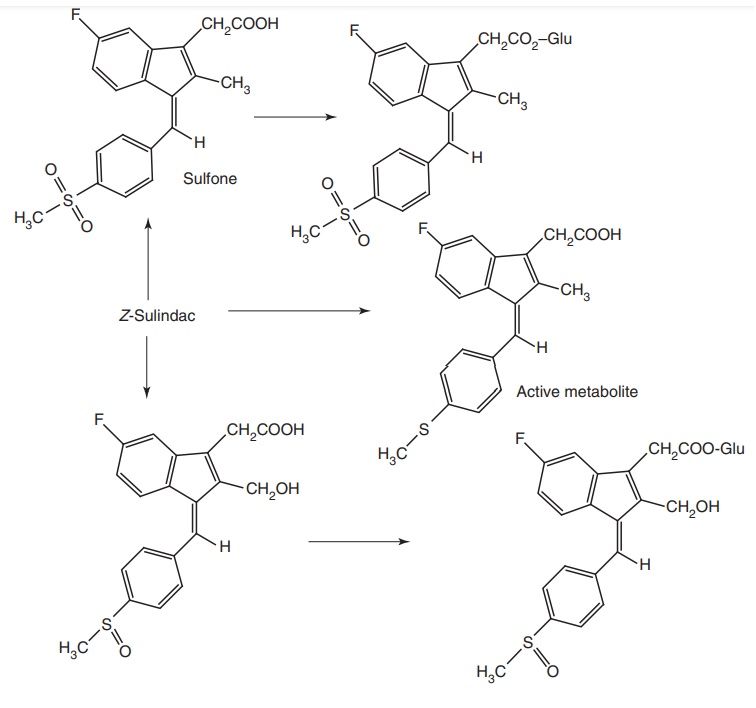
Metabolism: It is a prodrug to form active metabolites of sulphite. In
addition to it, sulindac is oxidized to corresponding sulphone and other
sulphone-glucuronide conjugates.
Properties and uses: Suindac is a yellow crystalline powder, very
slightly soluble in water, soluble in methylene chloride, and dilute solutions
of alkali hydroxides, sparingly soluble in alcohol. The (Z) isomer of sulindac
showed much more potent anti-inflammatory activity than the corresponding
(E)-isomer. The more polar and inactive sulphoxide is virtually the only form
excreted. It has analgesic, antipyretic, and anti-inflammatory properties. It is
usually employed in the treatment of rheumatic and muscular skeletal disorders,
acute gouty arthritis, and osteoarthritis.
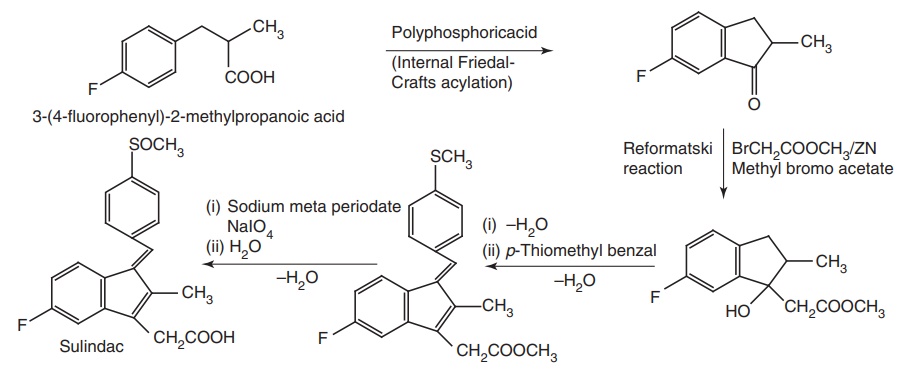
Synthesis
Route I. From: 3-(4-fluorophenyl)-2-methyl
propanoic acid
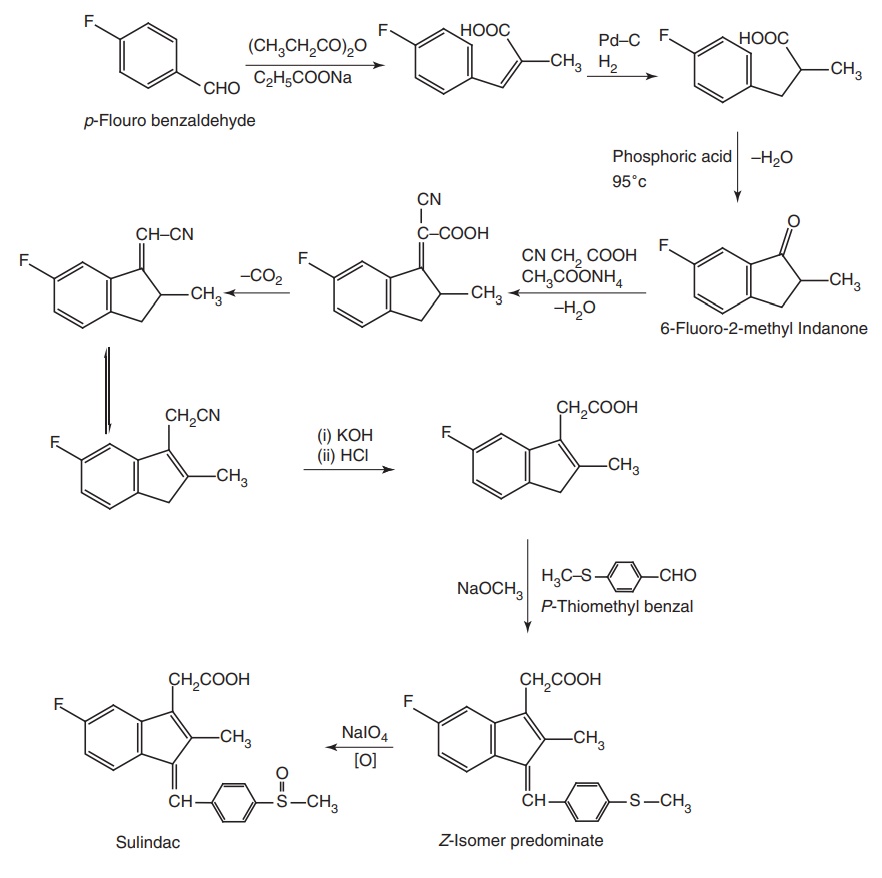
Synthesis
Route II. From p-Fluoro benzaldehyde
Assay: Dissolve the sample in methanol and titrate against 0.1 M sodium
hydroxide. Determine the end point potentiometrically.
Dose: Usual adult oral dose is 150 mg twice a day with food.
Dosage forms: Sulindac tablets B.P.
SAR of Indole Acetic Acid Derivatives
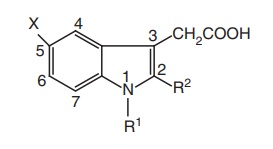
1. Placement
of other acidic functionalities instead of the carboxyl group decreases
activity and the amide derivatives are inactive.
2. Substituents of R1 useful for increasing anti-inflammatory activity are ranked as C6H4CH2 > alkyl > H.
3. Acylation
of the indole nitrogen with aryl/alkyl carboxylic acids results in the decrease
of activity.
4. Presence
of substituents on the N-benzoyl
derivatives in the p-position with F,
Cl, CF3, or S-CH3 groups provide greatest activity.
5. X
substituents activity are ranked as 5-OCH3 > N (CH3)2
> CH3 > H.
6. The presence of indole ring nitrogen is not essential for activity
because the corresponding 1-benzylidenylindene analogue (sulindac) is also
active.
7. Alkyl
groups especially methyl group at 2nd position is much active than aryl
substituted analogues.
8. Substitution
of a methyl group at the α position of the acetic acid side chain leads to
equiactive analogues.
9. Anti-inflammatory
activity was displayed only by the dextrorotatory enantiomer with similar
absolute configuration; it has 25 times the activity of phenylbutazone.
SAR of Pyrrole Acetic Acid Derivative
c. Pyrrole acetic acid derivative
Replacement
of the p-tolyl group with a p-chloro benzoyl moiety produced little
effect on activity, whereas introduction of a methyl group in the 4th position
and 5-p-chloro benzoyl analogues
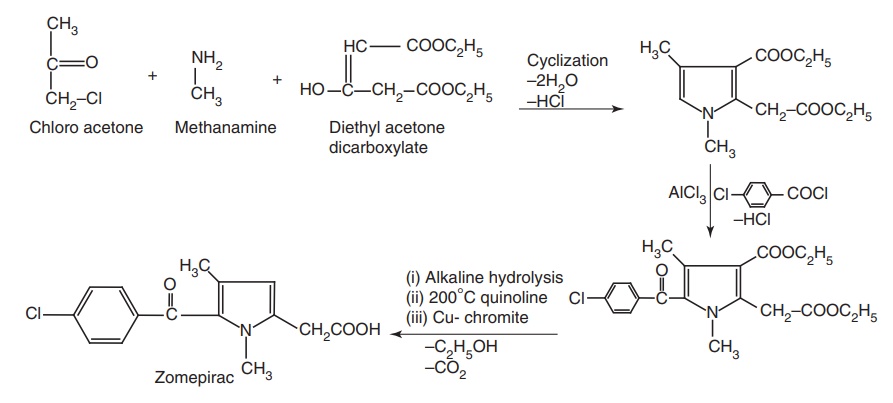
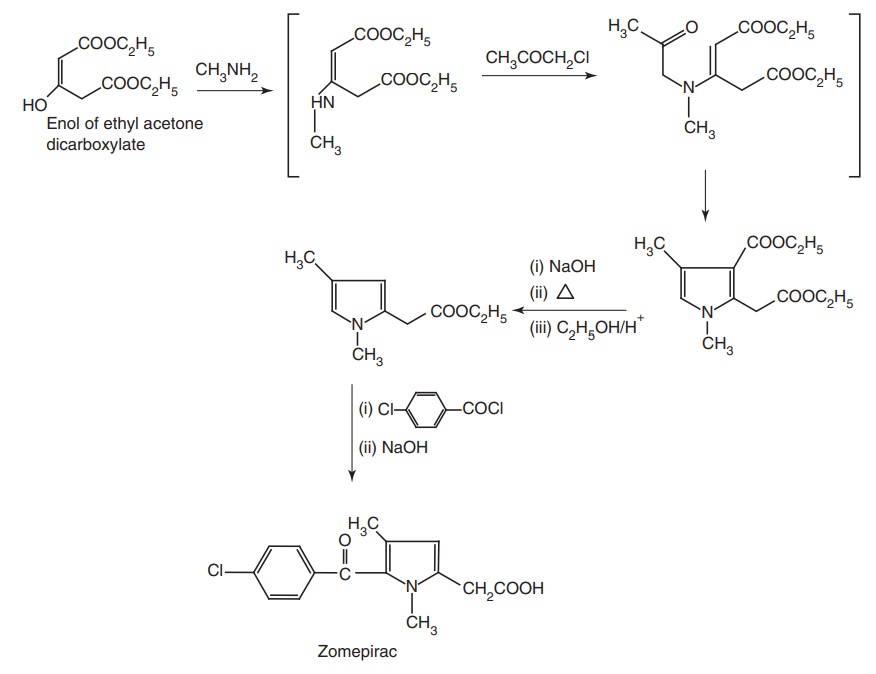
(zomeapirac) proved to be four times potent as tolmetin.
i. Tolmetin Sodium (Tolectin)
From: 1-Methyl pyrrole
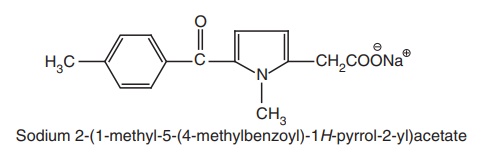
Synthesis
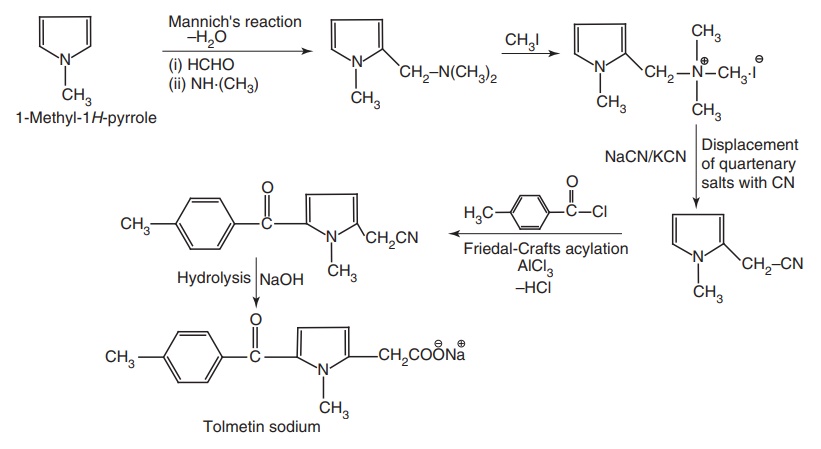
Metabolism: It is metabolized extensively first pass, involving hydroxylation
of p-methyl group to primary alcohol,
which is subsequently oxidized to dicarboxylic acid.
Properties and uses: It is a light yellow, crystalline powder,
soluble in water, slightly soluble in alcohol. It has antipyretic, analgesic,
and anti-inflammatory actions. It is employed in the treatment of rheumatic and
musculoskeletal disorders. The drug is, however, comparable to indomethacin and
aspirin in the control and management of disease activity.
Dose: Adult oral dose initially is 400 mg three times a day,
subsequently adjusted as per patient’s response.
ii. Zomepirac (Zomax)
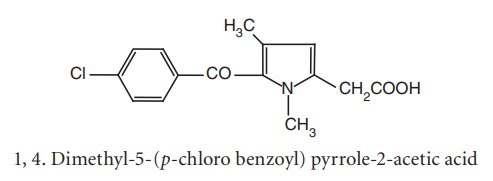
Properties and uses: A greater degree of analgesia for severe pain is
claimed for Zomepirac. It is used as an analgesic and an ant-inflammatory drug.
It is four times as potent as tolmetin.
Dose: Dose is 400 to 600 mg of zomepirac daily (zomepirac sodium 1.2 g
is approximately equivalent to 1 g of zomepirac).
Synthesis
Route I. From Chloro acetone
Route II. From: Enol of ethyl acetone
dicarboxylate
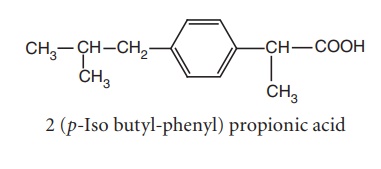
d. Aryl and heteroaryl acetic/propionic acid derivatives
i. Ibuprofen (Brufen, Motrin)
Synthesis
Route I. From Isobutyl benzene
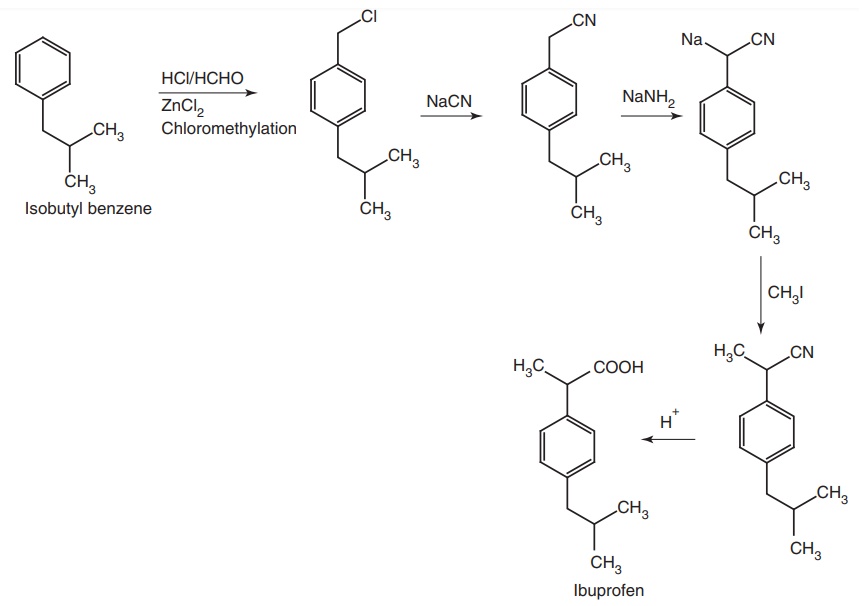
Route II. From: Isobutyl benzene
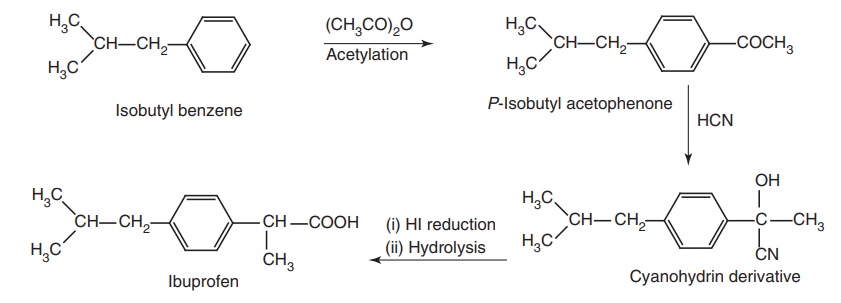
Metabolism: Oxidative metabolite of ibuprofen and unchanged drugs are
excreted in urine. Oxidation involves ω, ω , and ω oxidation of the para
isobutyl side chain, followed by alcohol oxidation, resulting from ω oxidation
to corresponding carboxylic acid.
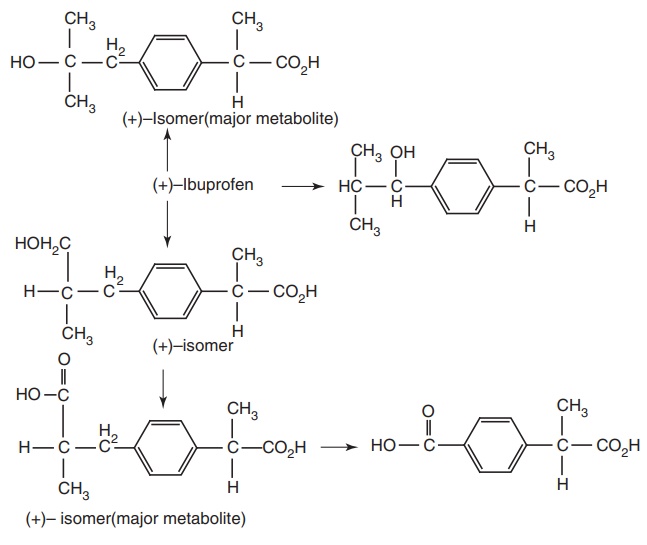
Properties and uses: Ibuprofen is a white crystalline powder or
colourless crystals, practically insoluble in water, soluble in acetone,
methanol, methylene chloride, and dilute solutions of alkali hydroxides and
carbonates. The precursor Ibufenac, which was abandoned owing to
hepatotoxicity, was less potent. Moreover, the activity resides in the (s)–(+)
isomer, not only in Ibuprofen but also throughout the arylacetic acid series.
Furthermore, these isomers are the more potent inhibitors of PG synthetase. It
is an anti-inflammatory drug that possesses antipyretic and analgesic action and
is used for the treatment of rheumatoid arthritis and osteoarthritis.
Assay: Dissolve the sample in methanol and titrate against 0.1 M sodium
hydroxide using phenolphthalein as indicator, until red colour is obtained.
Perform a blank titration
Dose: Usual oral adult dose as an analgesic (dysmenorrhoea) is 200–400
mg four to six times a day; in rheumatoid arthritis and osteoarthritis. The
dose is 300–400 mg three or four times a day.
Dosage forms: Ibuprofen tablets I.P., B.P, Ibuprofen cream B.P., Ibuprofen gel
B.P., Ibuprofen oral suspension B.P.
ii. Ibufenac
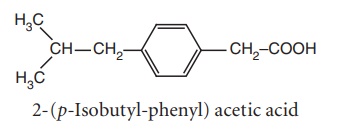
Synthesis
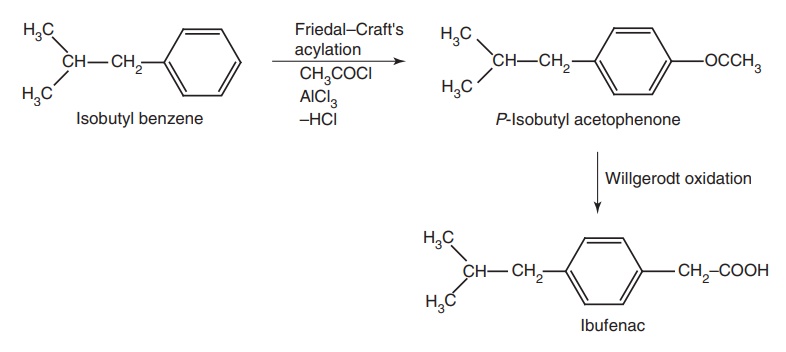
Properties and uses: It was formerly employed in the rheumatic
conditions, but was found to cause hepatotoxicity. It has analgesic, antipyretic,
and anti-inflammatory actions.
iii. Diclofenac (Voltaren, Voveran)
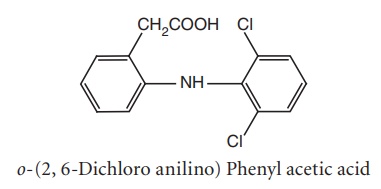
Metabolism: There are four major metabolites that are produced by aromatic
hydroxylation, that is, 4-hydroxy derivative, 5-hydroxy, 3-hydroxyl, and
4,5-dihydroxy metabolites. Remaining metabolites are excreted as sulphate
conjugates.
Properties and uses: Diclofenac sodium is a white or slightly
yellowish crystalline slightly hygroscopic powder, sparingly soluble in water,
soluble in methanol and alcohol, slightly soluble in acetone. Used in the
treatment of rheumatic arthritis.
Assay: Dissolve the sample in anhydrous acetic acid and titrate against
0.1 M perchloric acid. Determine the end point potentiometrically.
Dose: The usual dose is 20–50 mg three times a day. It can also be
given as a suppository.
Dosage forms: Diclofenac tablets I.P., Diclofenac injection I.P., Prolonged-release diclofenac tablets B.P., Gastro-resistant diclofenac tablets B.P., Prolonged-release diclofenac injection B.P., Prolonged-release diclofenac capsules B.P.
Synthesis
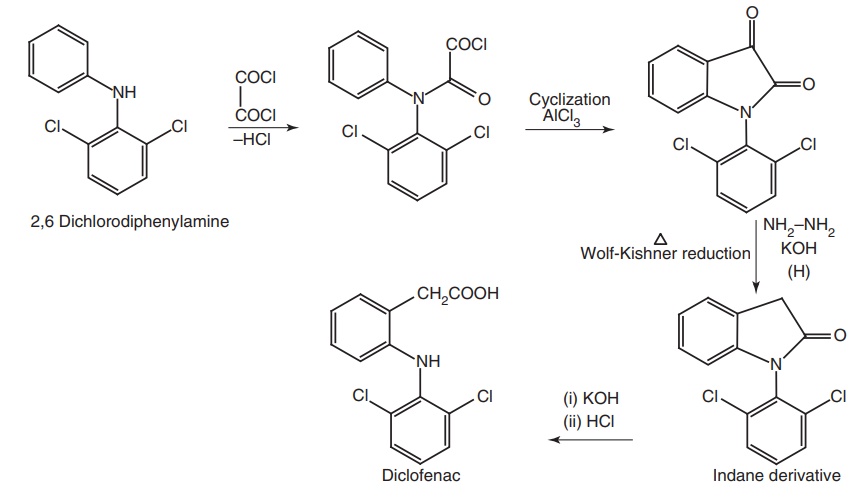
iv. Naproxen (Naprosyn)
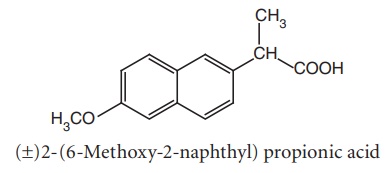
Metabolism: It is converted to 6-O-desmethyl
metabolite and then to glucuronide conjugate.
Properties and uses: Naproxen is a white crystalline powder,
practically insoluble in water, soluble in ethanol and in methanol. The drug is
fairly comparable to aspirin both in the management and control of disease
symptoms. Nevertheless, it has relatively lesser frequency and severity of
nervous system together with milder GI-effects. It possesses analgesic,
anti-inflammatory, and antipyretic actions, and it is used in the treatment of
rheumatic arthritis, dysmenorrhea, and acute gout.
Assay: Dissolve the sample in a mixture of water and methanol (1:3) and
titrate against 0.1 M sodium hydroxide, using 1 ml of phenolphthalein solution
as indicator.
Dose: For adult in rheumatoid arthritis, 250–375 mg as initial dose
two times a day; in acute gout, 750 mg as loading dose followed by 250 mg three
times a day until relieved.
Dosage forms: Naproxen oral suspension B.P., Naproxen suppositories B.P., Naproxen tablets B.P., Gastroresistant naproxen tablets B.P.
Synthesis
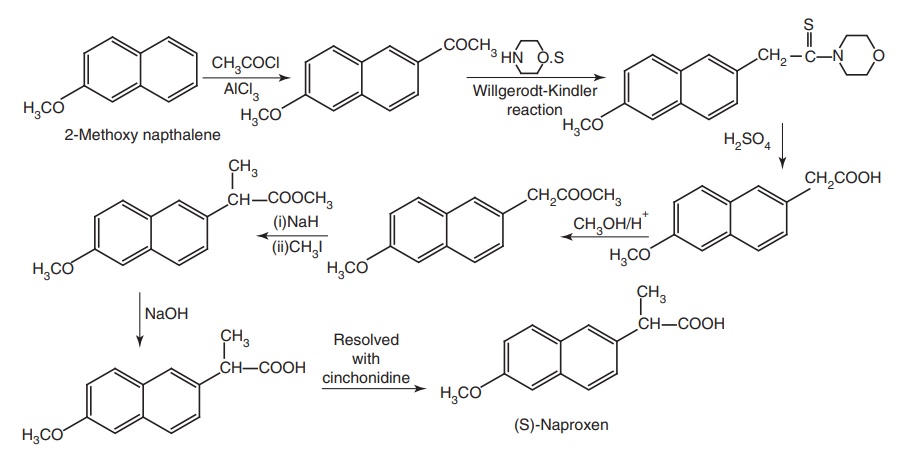
v. Fenoprofen (Nalton)
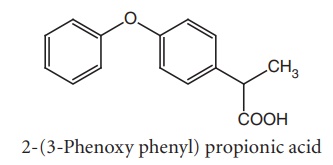
Synthesis
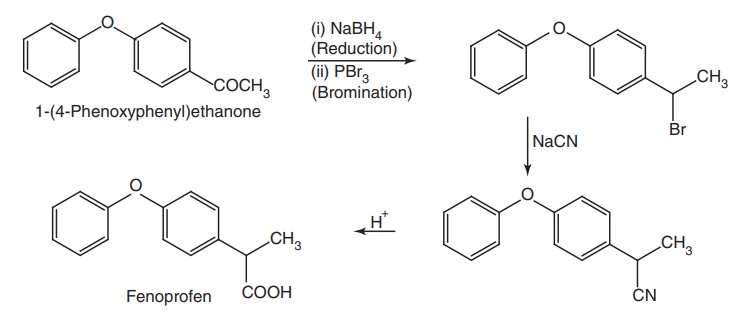
Metabolism: It is metabolized through glucuronide conjugation with a parent
drug and CYP2C9 to 4-hydroxy metabolites.
Properties and uses: Fenoprofen calcium is a white crystalline
powder, slightly soluble in water and soluble in ethanol. Fenoprofen calcium
has anti-inflammatory (antiarthritic) and analgesic properties. It has been
shown to inhibit PG synthetase. It is known to reduce joint-swelling, decrease
the duration of morning stiffness, and relieve pain. It is also indicated for
acute flares and exacerbations and in the long-term management of osteoarthritis
and rheumatoid arhrtitis.
Assay: Dissolve the sample in anhydrous acetic acid and titrate against
0.1 M perchloric acid. Determine the end point potentiometrically. Perform a
blank titration.
Dose: Dose is 50–100 mg twice daily with food.
Dosage forms: Fenoprofen tablets B.P.
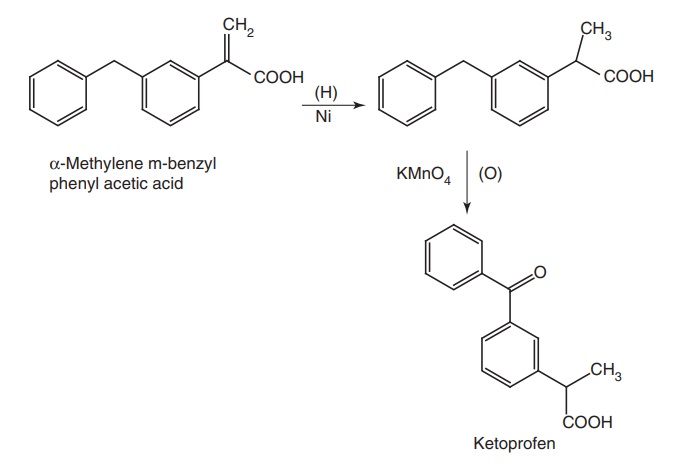
vi. Ketoprofen (Orudis)
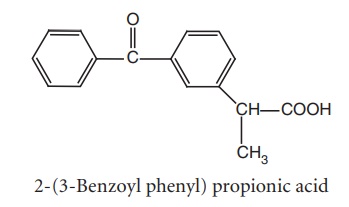
Synthesis
Route I. From:α-Methylene substituted m-benzyl phenyl acetic acid
Route II. From: 2-(4-Aminophenyl) propanoic acid
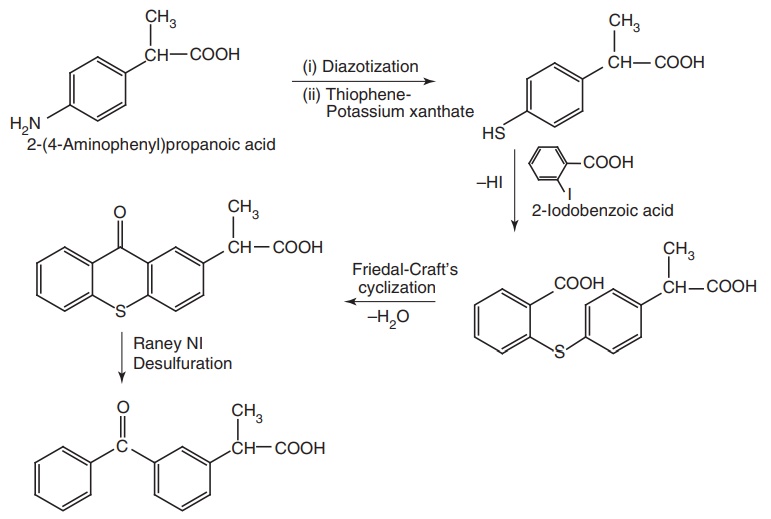
vii. Ketoprofen
Metabolism: It is metabolized by glucuronidation of carboxylic acid, CYP3A4,
and CYP2C9 hydroxylation of benzoyl ring and reduction of keto function.
Properties and uses: Ketoprofen is a white crystalline powder,
practically insoluble in water, soluble in acetone, in ethanol, and in
methylene chloride. It is closely related to fenoprofen in structure,
properties, and indications and has a low incidence of side effects and has
been approved for counter sale. It is used in the treatment of rheumatoid
arthritis and osteoarthritis
Assay: Dissolve the sample in ethanol and dilute with water and titrate
against 0.1 M sodium hydroxide. Determine the end point potentiometrically.
Dose: Usual adult oral dose for rheumatoid arthritis is 600 mg four
times daily; for osteoarthritis the dose is 300–600 mg four times a day.
Dosage forms: Ketoprofen capsules I.P., B.P., Ketoprofen gel B.P.
viii. Flurbiprofen (Ansaid)
Synthesis
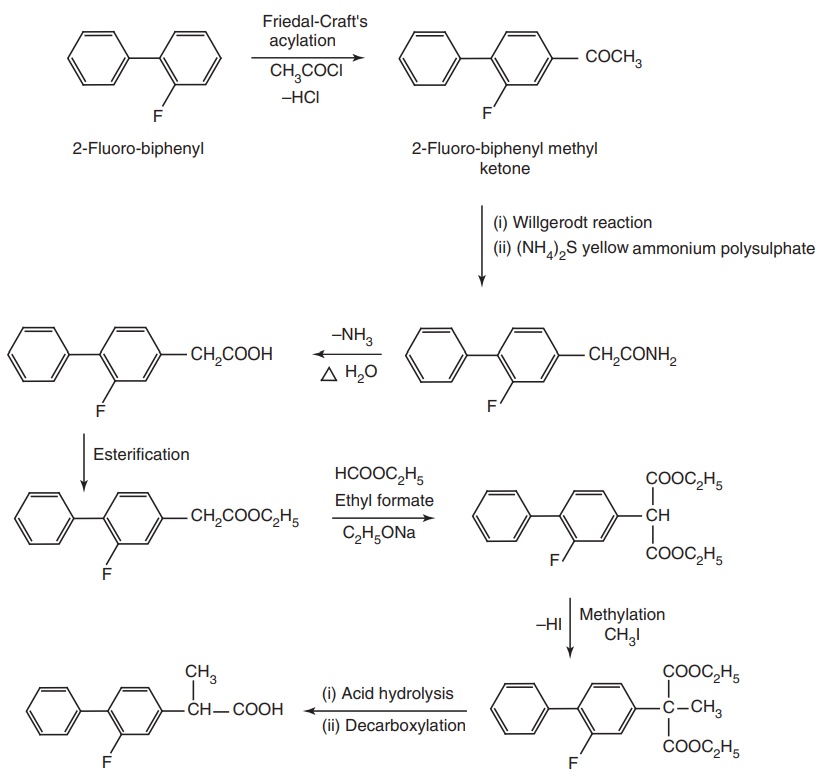
Properties and uses: Flurbiprofen is a white crystalline powder,
practically insoluble in water, soluble in alcohol, in methylene chloride, and
aqueous solutions of alkali hydroxides and carbonates. The drug is structurally
and pharmacologically related to fenoprofen, ibuprofen, and ketoprofen. Another
hydrotropic acid analogue that is used in the acute or long-term management of
rheumatoid arthritis and osteoarthritis, it posses analgesic, anti-inflammatory,
and antipyretic activities.
Assay: Dissolve the sample in alcohol and titrate against 0.1 M sodium
hydroxide. Determine the end point potentiometrically.
Dose: Usual adult dose is 150–200 mg a day in three to four divided
doses.
Dosage forms: Flurbiprofen tablets I.P., B.P, Flurbiprofen suppositories B.P.
viii. Caprofen
Synthesis
From: 1-(4-chlorophenyl) hydrazine
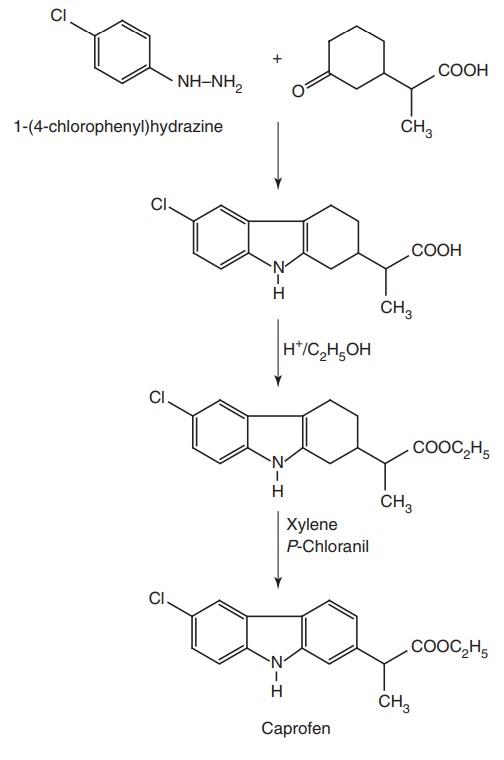
Uses: Used as an analgesic and anti-inflammatory agent.
ix. Ketorolac (Acular, Ketodrops, Ketlur)
Synthesis
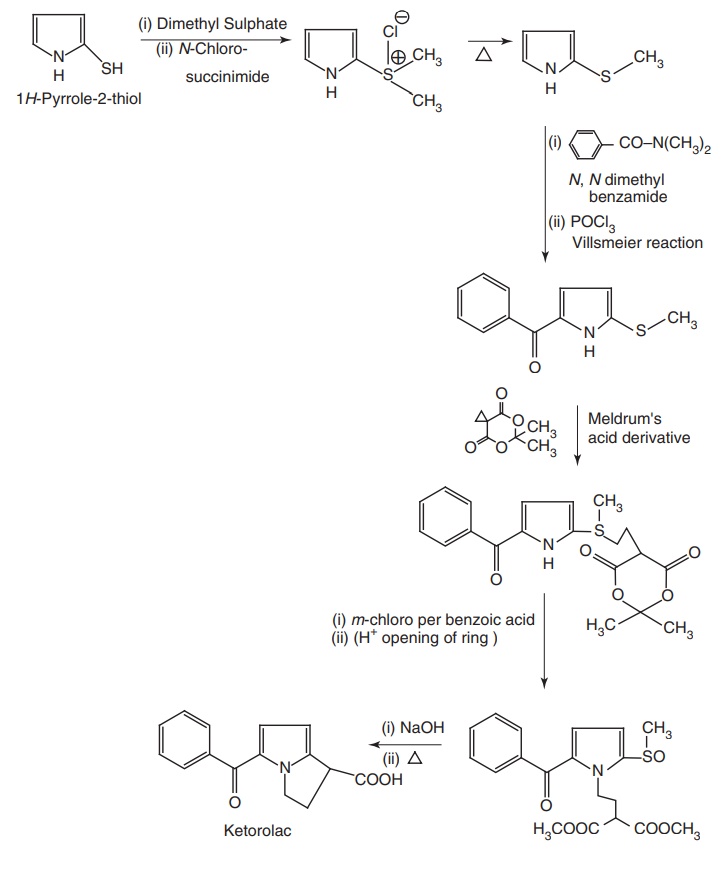
Properties and uses: Ketorolac is a white crystalline powder, soluble
in water and in methanol, slightly soluble in ethanol, practically insoluble in
methylene chloride. Ketorolac is a potent analgesic indicated for the treatment
of moderately severe and acute pain.
Assay: Dissolve the sample in anhydrous acetic acid and titrate against
0.1 M perchloric acid. Determine the end point potentiometrically.
Dose: The dose for ocular itching, which is associated with seasonal
allergic conjunctivitis, for reduction of ocular pain, and for photophobia in
patients undergoing incisional refractive sugery, instil one drop of a 0.5%
solution into the affected eyes four times daily.
x. Etodolac

Synthesis
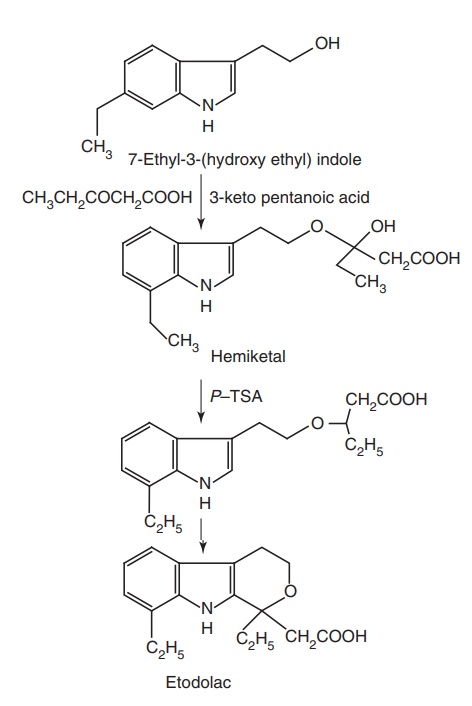
Metabolism: It is metabolized to 3-hydroxylated metabolite and to
glucuronide conjugates.
Properties and uses: Etodolac is a white crystalline powder,
practically insoluble in water, soluble in acetone and in ethanol. It has
anti-inflammatory activity and inhibits cyclooxygenase. It is used in the
treatment of osteoarthritis and rheumatoid arthritis. Gastrointestinal
irritation and ulceration is less with this drug than with other drugs.
Assay: Dissolve the sample in methanol and titrate against 0.1 M
tetrabutylammonium hydroxide. Determine the end point potentiometrically.
Perform a blank titration.
Dosage forms: Etodolac capsules B.P., Etodolac tablets B.P.
Related Topics
