Cardiac Glycosides
| Home | | Pharmacology |Chapter: Essential pharmacology : Cardiac Glycosides and Drugs for Heart Failure
These are glycosidic drugs having cardiac inotropic property. They increase myocardial contractility and output in a hypodynamic heart without a proportionate increase in O2 consumption.
CARDIAC GLYCOSIDES
These are glycosidic
drugs having cardiac inotropic property.
They increase myocardial contractility and output in a hypodynamic heart
without a proportionate increase in O2 consumption. Thus, efficiency
of failing heart is increased. In contrast, ‘cardiac
stimulants’ (Adr, theophylline) increase
O2 consumption rather disproportionately and tend to decrease
myocardial efficiency, i.e. increase in O2 consumption is more than
increase in contractility. Further, cardiac stimulants also increase heart rate
and have a short-lived action, while cardiac glycosides do not increase heart
rate and have a prolonged action.
William Withering, a
Birmingham physician, learnt that a decoction containing ‘foxglove’ ( Digitalis) with other herbals, prepared
by an old lady, relieved dropsy. He tried extract of foxglove alone and found
it to be remarkably effective in some cases. He published his classic monograph
‘An account of the Foxglove and some of its medicinal uses: with practical
remarks on dropsy and other diseases’ in 1785 and ascribed the beneficial
effect to an action on the kidney. Later Digitalis
was used indiscriminately, disregarding the precautions mentioned by Withering;
was found to be toxic and fell into disrepute. Cushney and Mackenzie, in the
beginning of 20th century, established its action on the heart and its use in
congestive heart failure (CHF). Strophanthus
was used as an arrow poison in Africa. Fraser discovered its digitalis like
action in 1890. The use of Squill has
come from Egyptian medicine, Toad skin
from Chinese medicine and Thevetin
from Unani medicine. Cases of poisoning with Thevetia and Convallaria
are occasionally seen.
By convention,
‘Digitalis’ is applied as a collective term for the whole group and has come to
mean ‘a cardiac glycoside’.
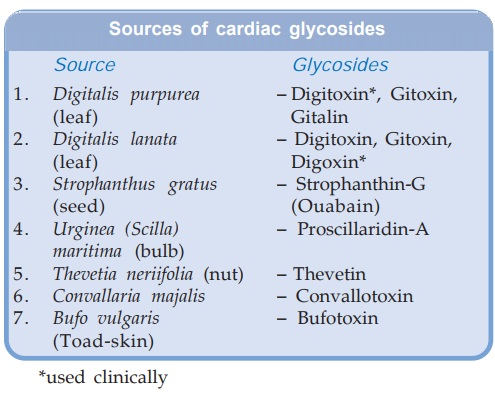
Chemistry
All are glycosides;
consist of an aglycone (genin) to
which are attached one or more sugar
(glucose or digitoxose) moieties. The pharmacological activity resides in the
aglycone, but attached sugars modify solubility and cell permeability. In
general, aglycones have shortlived and less potent action.
The aglycone consists
of a cyclopentanoperhydrophenanthrene (steroid) ring to which is attached a 5
or 6 membered unsaturated lactone ring. One or more hydroxyl and other substitutions
are present on the aglycone and determine its polarity, e.g. digoxigenin has an additional OH group
than digitoxigenin and is more polar.

Pharmacological Actions
All digitalis
glycosides have qualitatively similar action; there are only quantitative and
pharmacokinetic differences. Digoxin is described as prototype.
Heart
Digitalis has direct
effects on myocardial contractility and electrophysiological properties. In
addition, it has vagomimetic action, reflex effects due to alteration in haemodynamics
and direct CNS effects altering sympathetic activity.
Force of contraction Digitalis causes a
dose dependent increase in
force of contraction of heart—a positive inotropic action. This is especially
seen in the failing heart which is exquisitely sensitive. There is increased
velocity of tension development and higher peak tension can be generated.
Systole is shortened, diastole is prolonged. When a normal heart is subjected
to increased impedance to outflow, it generates increased tension so that
stroke volume is maintained upto considerably higher values of impedance (Fig.
37.1), while the failing heart is not able to do so and the stroke volume
progressively decreases. The digitalized failing heart regains some of its
capacity to contract more Forcefully when subjected to increased resistance to
ejection. There is more complete emptying of failing and dilated ventricles—cardiac
output is increased.
Digitalis increases force of contraction in normal heart as
well, but this is not translated into increased output, because the normal
heart empties nearly completely even otherwise and reduction of end diastolic
volume is counterproductive.
Tone It is defined by the
maximum length of the fibre at a given
filling pressure, or the resting tension in the muscle fibre. This is not
affected by therapeutic doses of digitalis. However, digitalis does decrease
end diastolic size of a failing ventricle, but this is a consequence of better
ventricular emptying and a reduction in filling pressure.
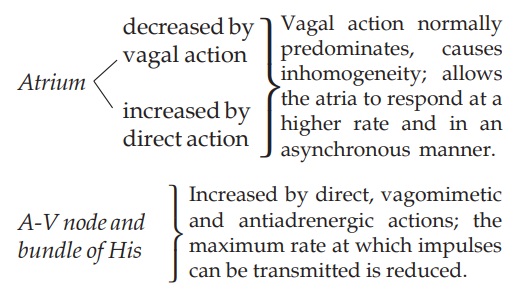
Rate Heart rate is
decreased by digitalis. Bradycardia is more
marked in CHF patients: improved circulation (due to positive inotropic action)
restores the diminished vagal tone and abolishes sympathetic overactivity. In
addition, digitalis slows the heart by vagal and extravagal actions.
Vagal tone is increased:
·
Reflexly through nodose ganglion and sensitization
of baroreceptors.
·
Direct stimulation of vagal centre.
·
Sensitization of SA node to ACh
Extravagal: A direct depressant
action on SA and AV nodes.
The vagal action manifests early and can be blocked by atropine, whereas the extravagal action becomes prominent later and cannot be reversed by atropine.
Electrophysiological Properties
The
electrophysiological effects of digitalis on different types of cardiac fibres
differ quantitatively and qualitatively. The Purkinje fibres, automatic and
conducting tissues are more sensitive. In addition to direct effects, the
indirect autonomic influences are important in the in situ heart.
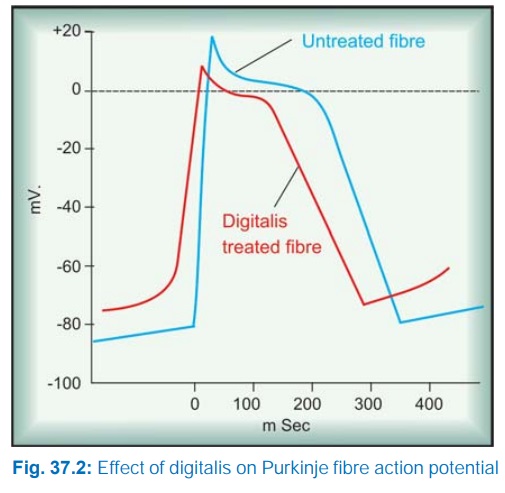
(a) Action potential
(AP ): The effects are illustrated diagrammatically in Fig. 37.2. The resting
membrane potential (RMP), is progressively decreased (shifted towards
isoelectric level) with increasing doses—excitability is enhanced at low doses
(due to reduction of gap between RMP and threshold potential) but depressed at
toxic doses (depolarization to below the level of critical potential which
inactivates the fast channels).
The rate of 0 phase depolarization is reduced. This action is
most marked in AV node and bundle of His.
The slope of phase4 depolarization is increased in the
PFs—ectopic automaticity is enhanced—latent
pacemakers become overt at high doses → extrasystoles. High doses of digitalis
produce coupled beats by another mechanism: the RMP shows oscillations during
phase-4; when their magnitude is sufficient enough, delayed
after-depolarizations result (see Fig. 38.1). The SA and A-V node automaticity
is reduced at therapeutic concentrations by vagal action which hyperpolarizes
these cells and reduces their phase-4 slope. Toxic doses markedly reduce RMP of
SA nodal cells by direct action and stop impulse generation.
(b) Effective
Refractory Period (ERP):
Ventricle—ERP is abbreviated by
direct action.
(c) Excitability:
Enhanced at low doses but depressed at high
doses as explained above.
(d) Conduction:
AV conduction is demonstrably slowed by therapeutic
doses due to a reduction in the rate of 0 phase depolarization. At high doses,
intraventricular conduction in PFs is also depressed by uncoupling of gap
junctions.
(e) ECG : Therapeutic doses of
digitalis produce changes in the ECG.
These are accentuated at high doses—may also produce arrhythmias. The changes
are:
Decreased amplitude or
inversion of T wave.
Increased PR interval
(slowing of AV conduction), AV block at toxic doses.
Shortening of QT
interval (reflecting shortening of systole).
Depression of ST
segment (at high doses— due to interference with repolarization).
The abnormal QRS of Wolff-ParkinsonWhite (WPW) syndrome is
widened because conduction through the normal AV bundle is slowed but not that
through the aberrant pathway.
Mechanism Of Action
Digitalis increases force of cardiac contraction
by a direct action independent of innervation. It selectively binds to
extracellular face of the membrane associated Na+K+ ATPase of myocardial fibres
and inhibitis this enzyme (Fig. 37.3). Inhibition of this cation pump results in
progressive accumulation of Na+ intracellularly. This indirectly results in
intracellular Ca2+ accumulation.
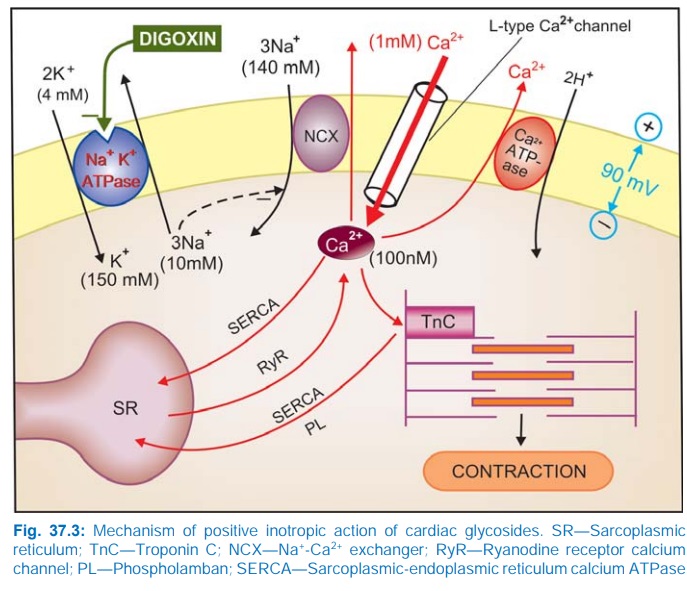
During depolarization Ca2+ ions enter the cell driven by the
steep Ca2+ gradient (>1 mM extracellular to < 100 nM cytosolic during
diastole) through voltage sensitive Ca2+ channels. This triggers release of
Ca2+ stored in sarcoplasmic reticulum (SR) → cytosolic Ca2+
increases transiently to about 500 nM (calcium transients) → triggers contraction.
Ca2+ is then actively taken up by SR and a fraction (equal to that which
entered from outside during depolarization) is extruded mainly by 3Na+/1Ca2+
exchange transporter (NCXantiporter) as well as by sarcolemmal Ca2+ pump (Ca2+
ATPase). During phase 3 of AP membrane Na+K+ATPase moves 3 intracellular Na+
ions for 2 extracellular K+ ions. The slight (1–1.5 mM) increase in cytosolic
Na+ over normal (8–10 mM) due to partial inhibition of Na+K+ATPase by digitalis
reduces transmembrane gradient of Na+ which drives the extrusion of Ca2+. The
excess Ca2+ remaining in cytosol is taken up into SR which progressively get
loaded with more Ca2+ → subsequent calcium transients are augmented.
The relationship of cytosolic [Na+] and [Ca2+] is such that a small percentage increase in Na+ concentration leads to a large percentage increase in Ca2+ concentration.
Moreover, raised
cytosolic Ca2+ induces greater entry of Ca2+ through voltage sensitive Ca2+
channels during the plateau phase. It has been shown that 1 mM rise in
cytosolic [Na+] results in 20–30% increase in the tension developed by
ventricular fibres.
Binding of glycoside to Na+K+ATPase is slow. Moreover, after
Na+K+ATPase inhibition, Ca2+ loading occurs gradually. As such, inotropic
effect of digitalis takes hours to develop, even after i.v. administration.
Inhibition of Na+K+
ATPase is clearly involved in the toxic actions of digitalis. At high doses,
there is depletion of intracellular K+; toxicity is partially reversed by
infusing K+. Excessive Ca2+ loading of SR results in spontaneous cycles of Ca2+
release and uptake producing oscillatory afterdepolarizations and aftercontractions.
Since both therapeutic and toxic effects of digitalis are due to myocardial
Ca2+ loading, these are inseparable and therapeutic index is low.
Blood Vessels
Digitalis has mild
direct vasoconstrictor
action—peripheral resistance is increased in normal individuals. However, in
CHF patients this is more than compensated by the indirect effect of
improvement in circulation, i.e. reflex sympathetic overactivity is withdrawn
and a net decrease in peripheral resistance occurs. Venous tone is improved in
normal individuals as well as in CHF patients.
Digitalis has no prominent effect on BP: systolic BP may
increase and diastolic may fall in CHF patients—pulse pressure increases.
Hypertension is no contraindication to the use of digitalis.
Despite a weak direct
coronary constrictor action, therapeutic doses of digitalis have no significant
effect on coronary circulation— coronary insufficiency is no contraindication
to its use. Coronary debt may even decrease if ventricles were in a dilated
state.
Kidney
Diuresis is seen
promptly in CHF patients, secondary to
improvement in circulation and renal perfusion. The retained salt and water is
gradually excreted. No diuresis occurs in normal individuals or in patients
with edema due to other causes.
CNS
Digitalis has little
apparent CNS effect in therapeutic dose.
Higher doses cause CTZ activation → nausea and vomiting. Still higher doses
produce hyperapnoea, central sympathetic stimulation, mental confusion,
disorientation and visual disturbances.
Pharmacokinetics
The pharmacokinetic
properties of digoxin and digitoxin are presented in Table 37.1.

Digitoxin is the most
lipid soluble, digoxin is relatively polar, while ouabain has the highest polar
character. Bioavailability of digoxin tablets from different manufacturers may
differ. Presence of food in stomach delays absorption of digoxin as well as
digitoxin.
The volume of distribution
of cardiac glycosides is large, e.g. 6–8 L/Kg in case of digoxin. All are
concentrated in the heart (~20 times than plasma), skeletal muscle, liver and
kidney.
Digitoxin is primarily
metabolized in liver, partly to digoxin, and undergoes some enterohepatic
circulation. Digoxin is primarily excreted unchanged by the kidney: mainly by
glomerular filtration; rate of excretion is altered parallel to creatinine
clearance. Its t½ is prolonged in elderly patients and in those with renal
insufficiency: dose has to be reduced. Dose of digitoxin is not greatly altered
in renal failure.
Cardiac glycosides are
cumulative drugs. When maintenance doses are given from the beginning, steady
state levels and full therapeutic effect are attained after 4 × t½, i.e. 6–7
days for digoxin and 4 weeks for digitoxin.
Preparations
1. Digoxin: DIGOXIN 0.25 mg tab.,
0.05 mg/ml pediatric elixir, 0.5 mg/2 ml inj. LANOXIN 0.25 mg tab, CARDIOXIN, DIXIN
0.25 mg tab, 0.5 mg/2 ml inj.
2. Digitoxin: DIGITOXIN 0.1 mg tab.
All glycosides have
the same safety margin; choice of preparation depends on kinetic properties. Digoxin is well absorbed orally, has
reasonably quick action, intermediate t½, dose adjustments are possible in 2–3
days, can be used for routine treatment as well as emergency; in case of
toxicity—discontinuation of the drug produces reasonably rapid disappearance of
manifestations. Thus, it is an all purpose and most commonly used glycoside.
Digitoxin may be used for
maintenance; because of its long t½,
diurnal fluctuations in blood level are low. However, any dose adjustment takes
weeks and toxic effects are more persistent. Therefore, most physicians prefer
digoxin for maintenance therapy also.
Adverse Effects
Toxicity of digitalis
is high, margin of safety is low (therapeutic index 1.5–3). Higher cardiac
mortality has been reported among patients with steadystate plasma digoxin
levels > 1.1 ng/ml during maintenance therapy. About 25% patients develop
one or other toxic symptom. The manifestations are:
Extracardiac: Anorexia, nausea,
vomiting and abdominal pain are
usually reported first: are due to gastric irritation, mesenteric
vasoconstriction and CTZ stimulation. Fatigue, no desire to walk or lift an
arm, malaise, headache, mental confusion, restlessness, hyperapnoea, disorienta
tion, psychosis and visual disturbances are the other complaints. Diarrhoea
occurs occasionally. Skin rashes and gynaecomastia are rare.
Cardiac: Almost every type of
arrhythmia can be produced by digitalis:
pulsus bigeminus, nodal and ventricular extrasystoles, ventricular tachycardia
and terminally fibrillation. Partial to complete AV block may be the sole
cardiac toxicity or it may accompany other arrhythmias. Severe bradycardia,
atrial extrasystoles, AF or AFl have also been noted. In about 2/3 patients
showing toxicity, extracardiac symptoms precede cardiac; in the rest serious
cardiac arrhythmias are the first manifestation. The central actions of
digitalis appear to contribute to the development of arrhythmias by inducing
fast and irregular activity in the cardiac sympathetic and vagus nerves.
Treatment
Further doses of digitalis must be stopped at the
earliest sign of toxicity; nothing more needs to be done in many patients,
especially if the manifestations are only extracardiac.
(a) For Tachyarrhythmias: When they are caused by chronic use of
digitalis and diuretics (both induce K+ depletion)—infuse KCl 20 m.mol/hour (max.
100 m. mol) i.v. or give orally in milder cases. K+ tends to antagonize
digitalis induced enhanced automaticity and decreases binding of the glycosides
to Na+K+ATPase by favouring a conformation of the enzyme that has lower
affinity for cardiac glycosides. When toxicity is due to acute ingestion of
large doses of digitalis, plasma K+ may be high; it should not be given from
outside. In any case, it is desirable to measure serum K+ to guide KCl therapy.
K+ is contraindicated if higher degree of AV block is present: complete AV
block and ventricular asystole can be precipitated.
(b) For Ventricular Arrhythmias: Lidocaine i.v. repeated as required is the drug of choice. It
suppresses the excessive automaticity, but does not accentuate AV block.
Phenytoin is also effective but seldom used now, because sudden deaths have
occurred when it was injected i.v. in digitalis intoxicated patients. Quinidine
and procainamide are contraindicated.
(c) For Supraventricular
Arrhythmias: Propranolol may be given i.v. or orally
depending on the urgency.
(d) For AV Block And Bradycardia: Atropine 0.6–1.2 mg i.m. may help; otherwise cardiac pacing is recommended.
Cardioversion by DC shock is contraindicated because severe
conduction defects may be unmasked in the digitalis intoxicated heart. Attempts
to enhance the elimination of digitalis by diuretics or haemodialysis are not
very effective.
Digoxin Antibody
Developed for
measuring plasma concentration of
digoxin by radioimmunoassay, it has been found effective in treating toxicity
as well. Digoxin specific antibody cross-reacts with digitoxin also. The Fab
fragment has been marketed in Europe as DIGIBIND (38 mg vial). It is
nonimmunogenic because it lacks the Fc fragment. Given by i.v. infusion it has
markedly improved the survival of seriously digitalis intoxicated patients. The
digoxin-Fab complex is rapidly excreted by kidney.
Precautions And Contraindications
·
Hypokalemia: enhances digitalis
toxicity by increasing its binding to
Na+K+ ATPase.
·
Elderly, renal or
severe hepatic disease: patients
are more sensitive.
· Myocardial infarction: severe arrhythmias
are more likely. Digitalis should be
used after MI only when heart failure is accompanied with AF and rapid ventricular
rate.
· WolffParkinsonWhite
syndrome: Digitalis is contraindicated—decreases
the ERP of bypass tract in 1/3 patients. In them rapid atrial impulses may be
transmitted to ventricles → VF may occur. Digitalis can increase the
chances of reentry by slowing conduction in the normal AV bundle and
accelerating it in the aberrant pathway.
Interactions
· Diuretics: cause hypokalemia
which can precipitate digitalis arrhythmias; potassium supplements may be given
prophylactically.
· Calcium: synergises with
digitalis →
precipitates toxicity.
·
Quinidine: reduces binding of
digoxin to tissue proteins as well as
its renal and biliary clearance by inhibiting efflux transporter Pglycoprotein → plasma concentration
is doubled → toxicity can occur. Verapamil, diltiazem, captopril and amiodarone: increase plasma
concentration of digoxin to variable
extents.
·
Adrenergic drugs: can induce
arrhythmias in digitalized patients;
both increase ectopic automaticity.
· Digoxin absorption can be reduced by metoclopramide (gastrointestinal
hurrying) and sucralfate which
adsorbs digoxin. Antacids, neomycin,
sulfasalazine also can reduce digoxin
absorption; stagger their administration.
Uses
The two main indications
of digitalis are CHF and control of ventricular rate in atrial fibrillation/flutter.
1. Congestive heart failure
CHF occurs when
cardiac output is insufficient to meet the demands of tissue perfusion. Heart
failure may primarily be due to systolic dysfunction or diastolic dysfunction.
Systolic Dysfunction The ventricles are dilated and unable to develop sufficient wall tension to eject adequate
quantity of blood. This occurs in ischaemic heart disease, valvular
incompetence, dilated cardiomyopathy, myocarditis, tachyarrhythmias.
Diastolic Dysfunction The ventricular wall is thickened and unable to relax properly during diastole;
ventricular filling is impaired because of which output is low. It occurs in
sustained hypertension, aortic stenosis, congenital heart disease, AV shunts, hypertrophic
cardiomyopathy.
However, most
patients, especially longstanding CHF, have both systolic and diastolic
dysfunction. Cardiac glycosides primarily mitigate systolic dysfunction. Best
results are obtained when myocardium is not primarily deranged, e.g. in
hypertension, valvular defects or that due to rapid heart rate in atrial
fibrillation. Poor response and more toxicity is likely when the myocardium has
been damaged by ischaemia, inflammation or degenerative changes and in thiamine
deficiency, as well as in high output failure (in anaemia).
Cardiac glycosides are
incapable of reversing the pathological changes of CHF or even arresting their
progress. Associated with hypertrophy, cardiac muscle undergoes remodeling
which may involve shift of isoforms of various functional proteins such as
myosin, creatine kinase, Na+K+ATPase, etc. Cardiac glycosides do not affect
remodeling.
Because of lower inotropic state, the failing heart is able to
pump much less blood at the normal filling pressure (Fig. 37.4), more blood
remains in the ventricles at the end of systole. The normal venous return is
added to it and Frank-Starling compensation is utilized to increase filling
pressure: the heart may be able to achieve normal stroke volume, but at a
filling pressure which produces congestive symptoms (venous engorgement, edema,
enlargement of liver, pulmonary congestion → dyspnoea, renal congestion
→ oliguria).
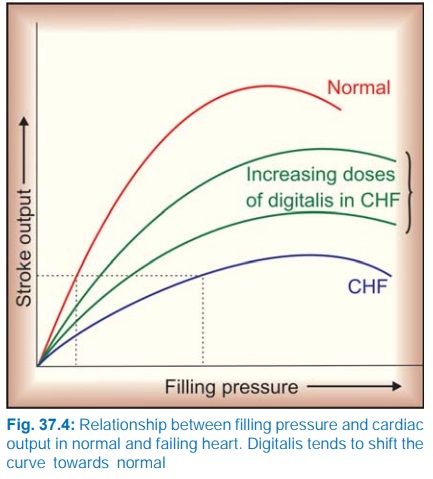
Digitalis induced enhancement of contractility increases ventricular
ejection and shifts the curve relating stroke output to filling pressure
towards normal, so that adequate output may be obtained at a filling pressure
that does not produce congestive symptoms. Improved tissue perfusion results in
withdrawal of sympathetic overactivity
heart rate and central venous pressure (CVP) are reduced.
Compensatory mechanisms retaining Na+ and water are inactivated → diuresis → edema is cleared.
Liver regresses, pulmonary congestion is reduced → dyspnoea abates,
cyanosis disappears. Low output symptoms like decreased capacity for muscular
work are mitigated.
A dilated ventricle automatically becomes inefficient according to
Laplace equation.
Wall tension = Intraventricular Pressure × Ventricular Radius
i.e. to generate the same ejection pressure a dilated ventricle
has to develop higher wall tension. By reducing end diastolic volume (due to
better emptying), digitalis restores efficiency of translation of cardiac work
into cardiac output. That is why O2 consumption does not increase
proportionately.
Dosage The dosing schedule
and route depend on the desired speed
of action and the factors which govern individual susceptibility. Generally, higher
dose is needed for more severe CHF.
There is some recent evidence that maintenance therapy with submaximal
inotropic doses (producing steady stage digoxin levels < 1 ng/ml) may
benefit by counteracting neurohumoral activation of CHF without risk of
toxicity.
Slow Digitalization In most mild to moderate cases, maintenance dose of digoxin (0.125–0.25
mg/day) is given from the beginning. Full response takes 5–7 days to develop,
but the procedure is much safer. In case adequate response is not seen after 1
week, increase the dose to 0.375 and then to 0.5 mg after another week.
Evaluation of adequate response is primarily clinical. Relief of signs and
symptoms of failure, reduction of heart rate and body weight to normal are the
best guide. Bradycardia (HR < 60/min) is an indication for stopping further
medication. ECG changes are not valuable in quantitation of doses unless
arrhythmias occur.
Rapid Oral
Digitalization Digoxin 0.5–1.0 mg stat followed by 0.25 mg every 6 hours with careful
monitoring and watch for toxicity till response occurs—generally takes 6–24
hours (total dose 0.75–1.5 mg). This is seldom practised now.
Emergent I.V. Digitalization It is practised
rarely now, only as a desperate measure in CHF or in atrial fibrillation.
Digoxin 0.25 mg followed by 0.1 mg hourly is given by slow i.v. injection with
close ECG, BP and CVP monitoring till response occurs (2–6 hours, total dose
0.5–1.0 mg).
Current status of digitalis Before the introduction
of high ceiling diuretics and ACE inhibitors, digitalis was considered an
indispensible part of anti-CHF treatment. It is not so now. Many mild-to-moderate
cases can be managed without digitalis, i.e. with diuretics and vasodilators,
especially an ACE inhibitor. Lately, β blockers have got added to the standard
therapy. Emergency i.v. use of digoxin for CHF is practically extinct. However,
digitalis is still the most effective drug capable of restoring cardiac
compensation, especially in patients with dilated heart and low ejection fraction;
all patients not controlled by ACE inhibitor/AT1 receptor blocker, blocker
and diuretic should be treated with digitalis. Uncertainty exists in the area
of maintenance therapy, i.e. after decompensation has been corrected in
patients not having atrial fibrillation (AF). There has been a trend to
discontinue digitalis once compensation has been restored, especially in mild-to-moderate
cases.
Two large randomized
trials—Randomized assessment of digoxin on inhibition of angiotensin converting
enzyme (RADIANCE, 1993) and Prospective randomized study of ventricular failure
and efficacy of digoxin (PROVED, 1993) on CHF patients in sinus rhythm showed
that discontinuation of digitalis resulted in reduced exercise capacity and
haemodynamic deterioration in a significant number of cases despite continued
use of diuretic with or without ACE inhibitor. A trend has emerged in favour of
maintenance ACE inhibitor and digitalis therapy with intermittent symptom based
use of diuretics. However, the trials referred above also showed that digitalis
can be withdrawn without haemodynamic deterioration in 60% (not receiving ACE
inhibitor) and in 72% (receiving ACE inhibitor) patients.
If stable clinical
state has been maintained for 2–3 months, withdrawal of digitalis may be attempted.
Early reinstitution of digitalis is recommended if cardiac status declines.
Continued digitalis therapy is the best course in CHF patients with atrial
fibrillation.
Large studies
including those by Digoxin Investigation Group (DIG) have found no evidence
that digitalis decreases overall mortality in CHF patients, though episodes of
decompensation and heart failure deaths are reduced. The two major limitations
in the use of cardiac glycosides are low margin of safety and inability to reverse/retard
the processes which cause the heart to fail.
2. Cardiac Arrhythmias
Atrial Fibrillation (AF) Digitalis is the drug
of choice for controlling
ventricular rate in AF, whether associated with CHF or not. However, it is
incapable of curing AF, i.e. does not revert it to sinus rhythm, even
perpetuates it.
Digitalis reduces ventricular rate in AF by decreasing the
number of impulses that are able to pass down the AV node and bundle of His.
It increases ERP of AV node by direct, vagomimetic and antiadrenergic
actions: the minimum interval between consecutive impulses that can
successfully traverse the conducting tissue is increased.
A degree of AV block is naturally established in AF. Because of
the relatively long ERP of AV node, many of the atrial impulses (~500/min)
impinge on it while it is still refractory; others falling early in the
relative refractory period get extinguished by decremental conduction. These
concealed impulses, nevertheless, leave the upper margin of AV node refractory for
a further period. Thus, any influence which increases rate of AF, by itself
reduces ventricular rate. Digitalis decreases average atrial ERP and temporally
disperses it (vagal action), thereby increasing fibrillation frequency and
indirectly prolonging the interval between any two impulses that are
successfully conducted to the ventricle.
When digitalis is
given in AF, average ventricular rate decreases in a dose-dependent manner and
pulse deficit is abolished because ventricle does not receive an impulse very
early in diastole before it has had time to fill up reasonably. The therapeutic
endpoint can be clearly defined: the dose should be adjusted to a ventricular
rate of 70–80/min at rest. If this is not possible with digitalis alone, a β blocker or verapamil
may be added.
Atrial Flutter (AFI) The atrial rate is
200–350/ min (less than that in
AF), but atrial contractions are regular and synchronous. A variable degree of
AV block, depending on the mean ERP of AV node, is naturally established. Digitalis
enhances this AV block, reduces ventricular rate and prevents sudden shift of AV
block to a lower degree (as may occur during exercise or sympathetic
stimulation). Digitalis may convert AFl to AF by reducing atrial ERP and making
it inhomogeneous. This is a welcome response because control of ventricular
rate is easier in AF (graded response occurs) than in AFl (AV block shifts in
steps). In nearly ½ of the patients when digitalis is stopped, this induced AF
reverts to sinus rhythm since the cause of atrial inhomogeneity is gone.
Alternatively, AFl may be terminated by cardioversion/radiofrequency ablation
and its recurrence prevented by subsequent digitalis treatment.
Paroxysmal Supraventricular Tachycardia
(PSVT) It is a common arrhythmia with a rate 150–200/ min and 1 : 1 AV
conduction. It is mostly due to reentry involving the SA or AV node. Rigidly
circumscribed magnitudes of ERP and conduction velocity are required for its
persistence. A parenteral glycoside may be injected i.v.— increases vagal tone
and depresses the path through the SA/AV node, or the ectopic focus, and
terminates the arrhythmia (success in 1/3 cases). Verapamil/adenosine are more
effective, less toxic and act faster. Digitalis is now reserved for preventing
recurrences in selected cases.
Related Topics
