Cobalamin (Vitamin B12)
| Home | | Biochemistry |Chapter: Biochemistry : Vitamins
Vitamin B12 is required in humans for two essential enzymatic reactions: the remethylation of homocysteine (Hcy) to methionine and the isomerization of methylmalonyl coenzyme A (CoA), which is produced during the degradation of some amino acids (isoleucine, valine, threonine, and methionine) and fatty acids (FAs) with odd numbers of carbon atoms.
COBALAMIN (VITAMIN B12)
Vitamin B12
is required in humans for two essential enzymatic reactions: the remethylation
of homocysteine (Hcy) to methionine and the isomerization of methylmalonyl
coenzyme A (CoA), which is produced during the degradation of some amino acids
(isoleucine, valine, threonine, and methionine) and fatty acids (FAs) with odd
numbers of carbon atoms (Figure 28.5). When cobalamin is deficient, unusual
(branched) FAs accumulate and become incorporated into cell membranes,
including those of the central nervous system (CNS). This may account for some
of the neurologic manifestations of vitamin B12 deficiency. [Note:
Folic acid (as N5-methyl THF) is also required in the remethylation
of Hcy. Therefore, deficiency of B12 or folate results in elevated
Hcy levels.]
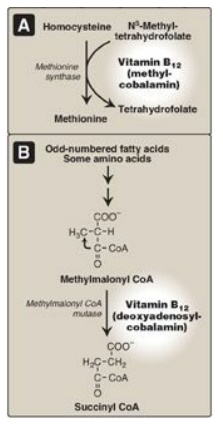
Figure 28.5 Reactions requiring coenzyme forms of vitamin B12. CoA = coenzyme A.
A. Structure of cobalamin and its coenzyme forms
Cobalamin contains a corrin ring system that resembles the porphyrin ring of heme, and but differs in that two of the pyrrole rings are linked directly rather than through a methene bridge. Cobalt is held in the center of the corrin ring by four coordination bonds with the nitrogens of the pyrrole groups. The remaining coordination bonds of the cobalt are with the nitrogen of 5,6-dimethylbenzimidazole and with cyanide in commercial preparations of the vitamin in the form of cyanocobalamin (Figure 28.6). The physiologic coenzyme forms of cobalamin are 5 - deoxyadenosylcobalamin and methylcobalamin, in which cyanide is replaced with 5-deoxyadenosine or a methyl group, respectively (see Figure 28.6).
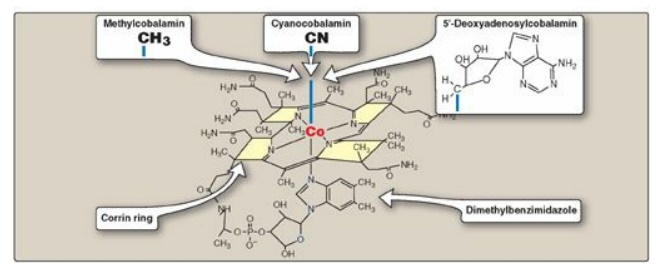
Figure 28.6 Structure of
vitamin B12 (cyanocobalamin) and its coenzyme forms (methylcobalamin
and 5 -deoxyadenosylcobalamin).
B. Distribution of cobalamin
Vitamin B12
is synthesized only by microorganisms, and it is not present in plants. Animals
obtain the vitamin preformed from their natural bacterial flora or by eating
foods derived from other animals. Cobalamin is present in appreciable amounts
in liver, red meat, fish, eggs, dairy products, and fortified cereals.
C. Folate trap hypothesis
The effects of
cobalamin deficiency are most pronounced in rapidly dividing cells, such as the
erythropoietic tissue of bone marrow and the mucosal cells of the intestine.
Such tissues need both the N5,N10-methylene and
N10-formyl forms of THF for the synthesis of nucleotides required for DNA
replication. However, in vitamin B12 deficiency, the utilization of
the N5-methyl form of THF in the B12-dependent
methylation of homocysteine to methionine is impaired. Because the methylated
form cannot be converted directly to other forms of THF, folate is trapped in
the N5-methyl form, which accumulates. The levels of the other forms
decrease. Thus, cobalamin deficiency leads to a deficiency of the THF forms
needed in purine and TMP synthesis, resulting in the symptoms of megaloblastic
anemia.
D. Clinical indications for vitamin B12
In contrast to other
water-soluble vitamins, significant amounts (2–5 mg) of vitamin B12
are stored in the body. As a result, it may take several years for the clinical
symptoms of B12 deficiency to develop as a result of decreased
intake of the vitamin. [Note: Deficiency happens much more quickly if
absorption is impaired (see below).] B12 deficiency can be
determined by the level of methylmalonic acid in blood, which is elevated in
individuals with low intake or decreased absorption of the vitamin.
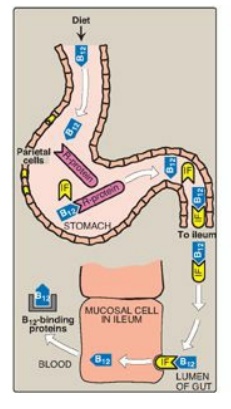
Figure 28.7 Absorption of
vitamin B12. IF = intrinsic factor.
1. Pernicious anemia: Vitamin B12 deficiency
is most commonly seen in patients who fail to absorb the vitamin from the
intestine. B12 is released from food in the acidic environment of
the stomach. [Note: Malabsorption of cobalamin in the elderly is most often due
to reduced secretion of gastric acid (achlorhydria).] Free B12 then
binds a glycoprotein (R-protein), and the complex moves into the intestine. B12
is released from the R-protein by pancreatic enzymes and binds another
glycoprotein, intrinsic factor (IF). The cobalamin–IF complex travels through
the intestine and binds to specific receptors on the surface of mucosal cells
in the ileum. The cobalamin is transported into the mucosal cell and,
subsequently, into the general circulation, where it is carried by its binding
protein (transcobalamin). B12 is taken up and stored in the liver,
primarily. It is released into bile and efficiently reabsorbed in the ileum.
Severe malabsorption of vitamin B12 leads to pernicious anemia. This
disease is most commonly a result of an autoimmune destruction of the gastric
parietal cells that are responsible for the synthesis of IF (lack of IF
prevents B12 absorption). [Note: Patients who have had a partial or
total gastrectomy become IF deficient and, therefore, B12
deficient.] Individuals with cobalamin deficiency are usually anemic, and they
show neuropsychiatric symptoms later, as the disease develops. The CNS effects
are irreversible and occur by mechanisms that appear to be different from those
described for megaloblastic anemia. Pernicious anemia requires life-long
treatment with either high-dose oral B12 or intramuscular injection
of cyanocobalamin. [Note: Supplementation works even in the absence of IF
because approximately 1% of B12 uptake is by IF-independent
diffusion.]
Folic acid supplementation can partially reverse the hematologic abnormalities of B12 deficiency and, therefore, can mask a cobalamin deficiency. Thus, to prevent the CNS effects of B12 deficiency, therapy for megaloblastic anemia is initiated with both vitamin B12 and folic acid until the cause of the anemia can be determined.
