Components Required for Translation
| Home | | Biochemistry |Chapter: Biochemistry : Protein Synthesis
A large number of components are required for the synthesis of a protein.
COMPONENTS REQUIRED FOR TRANSLATION
A large number of
components are required for the synthesis of a protein. These include all the
amino acids that are found in the finished product, the mRNA to be translated,
transfer RNA (tRNA) for each of the amino acids, functional ribosomes, energy
sources, and enzymes as well as noncatalytic protein factors needed for the
initiation, elongation, and termination steps of polypeptide chain synthesis.
A. Amino acids
All the amino acids that eventually appear in the finished protein must be present at the time of protein synthesis. If one amino acid is missing, translation stops at the codon specifying that amino acid. [Note: This demonstrates the importance of having all the essential amino acids in sufficient quantities in the diet to ensure continued protein synthesis.]
B. Transfer RNA
At least one specific
type of tRNA is required for each amino acid. In humans, there are at least 50
species of tRNA, whereas bacteria contain at least 30 species. Because there
are only 20 different amino acids commonly carried by tRNA, some amino acids
have more than one specific tRNA molecule. This is particularly true of those
amino acids that are coded for by several codons.
1. Amino acid attachment site: Each tRNA molecule has an
attachment site for a specific (cognate) amino acid at its 3I -end (Figure
31.6). The carboxyl group of the amino acid is in an ester linkage with the
3-hydroxyl of the ribose portion of the adenine (A) nucleotide in the —CCA
sequence at the 3I -end of the tRNA. [Note: When a tRNA has a covalently
attached amino acid, it is said to be charged, and when it does not, it is said
to be uncharged. The amino acid attached to the tRNA molecule is said to be
activated.]
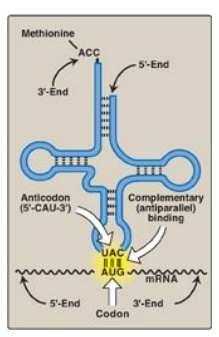
Figure 31.6 Complementary, antiparallel binding of the anticodon for methionyl-tRNA (CAU) to the messenger RNA (mRNA) codon for methionine (AUG), the initiation codon for translation.
2. Anticodon: Each tRNA molecule also contains a three-base nucleotide sequence, the anticodon, that pairs with a specific codon on the mRNA (see Figure 31.6). This codon specifies the insertion into the growing peptide chain of the amino acid carried by that tRNA.
C. Aminoacyl-tRNA synthetases
This family of enzymes
is required for attachment of amino acids to their corresponding tRNAs. Each
member of this family recognizes a specific amino acid and all the tRNAs that
correspond to that amino acid (isoaccepting tRNAs, up to five per amino acid).
Aminoacyl-tRNA synthetases catalyze a two-step reaction that results in the
covalent attachment of the carboxyl group of an amino acid to the 3I -end of
its corresponding tRNA. The overall reaction requires adenosine triphosphate
(ATP), which is cleaved to adenosine monophosphate (AMP) and inorganic
pyrophosphate (PPi) as shown in Figure 31.7. The extreme specificity of the
synthetases in recognizing both the amino acid and its cognate tRNA contributes
to the high fidelity of translation of the genetic message. In addition to
their synthetic activity, the aminoacyl-tRNA synthetases have a “proofreading”
or “editing” activity that can remove an incorrect amino acid from the enzyme
or the tRNA molecule.
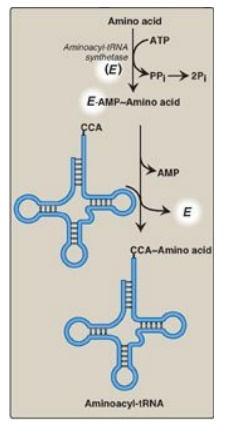
Figure 31.7 Attachment of a specific amino acid to its corresponding tRNA by aminoacyl-tRNA synthetase. PPi = pyrophosphate; Pi = inorganic phosphate; A = adenine; C = cytosine; ATP = adenosine triphosphate; AMP = adenosine monophosphate.
D. Messenger RNA
The specific mRNA
required as a template for the synthesis of the desired polypeptide chain must
be present. [Note: In eukaryotes, mRNA is circularized for use in translation.]
E. Functionally competent ribosomes
Ribosomes are large
complexes of protein and ribosomal RNA ([rRNA], Figure 31.8), in which rRNA
predominates. They consist of two subunits (one large and one small) whose
relative sizes are given in terms of their sedimentation coefficients, or S
(Svedberg) values. [Note: Because the S values are determined both by shape as
well as molecular mass, their numeric values are not strictly additive. For
example, the prokaryotic 50S and 30S ribosomal subunits together form a 70S
ribosome. The eukaryotic 60S and 40S subunits form an 80S ribosome.]
Prokaryotic and eukaryotic ribosomes are similar in structure, and serve the
same function, namely, as the macromolecular complexes in which the synthesis
of proteins occurs.
The small ribosomal subunit binds mRNA and is
responsible for the accuracy of translation by ensuring correct base-pairing
between the codon in the mRNA and the anticodon in the tRNA. The large
ribosomal subunit catalyzes formation of the peptide bonds that link amino acid
residues in a protein.
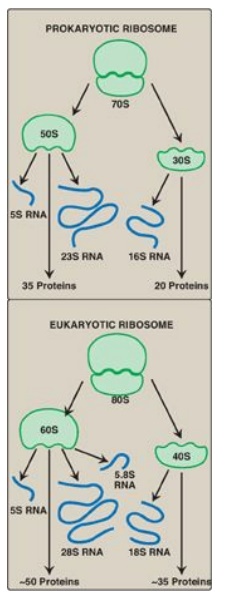
Figure 31.8 Ribosomal composition. [Note: The number of proteins in the eukaryotic ribosomal subunits varies somewhat from species to species.] S = Svedberg unit.
1. Ribosomal RNA: As discussed, prokaryotic ribosomes contain three
size species of rRNA, whereas eukaryotic ribosomes contain four (see Figure
31.8). The rRNAs are generated from a single pre-rRNA by the action of
ribonucleases, and some bases and riboses are modified.
2. Ribosomal proteins: Ribosomal proteins are present in
greater numbers in eukaryotic ribosomes than in prokaryotic ribosomes. These
proteins play a variety of roles in the structure and function of the ribosome
and its interactions with other components of the translation system.
3. A, P, and E sites on the ribosome: The ribosome has three binding sites for tRNA molecules: the A, P, and E sites, each of which extends over both subunits. Together, they cover three neighboring codons. During translation, the A site binds an incoming aminoacyl-tRNA as directed by the codon currently occupying this site. This codon specifies the next amino acid to be added to the growing peptide chain. The P-site codon is occupied by peptidyl-tRNA. This tRNA carries the chain of amino acids that has already been synthesized. The E site is occupied by the empty tRNA as it is about to exit the ribosome. (See Figure 31.13 for an illustration of the role of the A, P, and E sites in translation.)
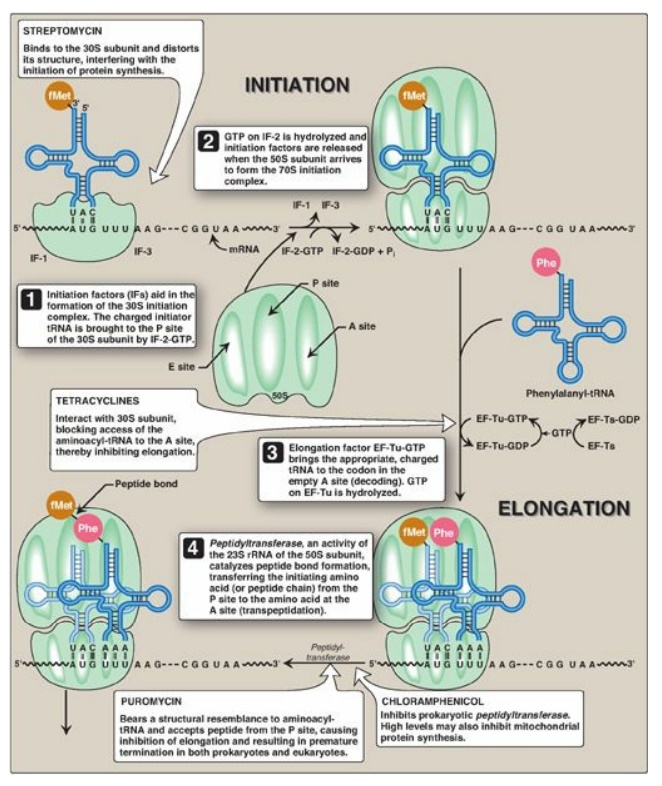
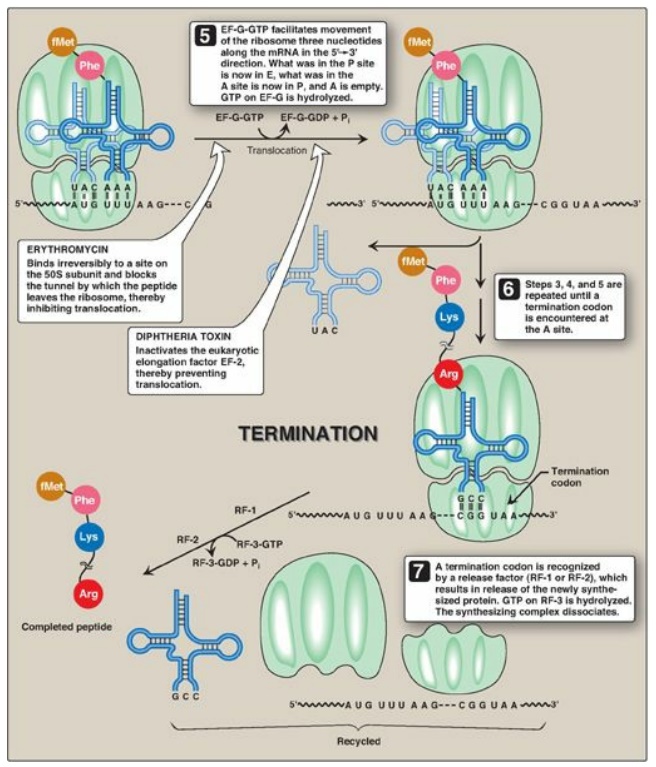
Figure 31.13 Steps in
prokaryotic protein synthesis (translation), and their inhibition by
antibiotics. [Note: EF-Ts is a guanine nucleotide exchange factor. It
facilitates the removal of GDP, allowing its replacement by GTP. The eukaryotic
equivalent is EF-1βγ.] fMet = formylated methionine; S = Svedberg unit; GTP =
guanine nucleoside triphosphate; Phe = phenylalanine.
4. Cellular location of ribosomes: In eukaryotic cells, the ribosomes
are either “free” in the cytosol or are in close association with the
endoplasmic reticulum (which is then known as the “rough” endoplasmic
reticulum, or RER). The RER-associated ribosomes are responsible for
synthesizing proteins that are to be exported from the cell as well as those
that are destined to become incorporated into plasma, endoplasmic reticulum, or
Golgi membranes or imported into lysosomes. Cytosolic ribosomes synthesize
proteins required in the cytosol itself or destined for the nucleus,
mitochondria or peroxisomes. [Note: Mitochondria contain their own set of
ribosomes and their own unique, circular DNA. Most mitochondrial proteins,
however, are encoded by nuclear DNA, synthesized in the cytosol, and
posttranslationally targeted to mitochondria.]
F. Protein factors
Initiation, elongation,
and termination (or release) factors are required for peptide synthesis. Some
of these protein factors perform a catalytic function, whereas others appear to
stabilize the synthetic machinery. [Note: A number of the factors are monomeric
G proteins, and thus are active when bound to guanosine triphosphate (GTP) and
inactive when bound to guanosine diphosphate (GDP).]
G. ATP and GTP are required as sources of energy
Cleavage of four
high-energy bonds is required for the addition of one amino acid to the growing
polypeptide chain: two from ATP in the aminoacyl-tRNA synthetase reaction—one
in the removal of PPi, and one in the subsequent hydrolysis of the PPi to
inorganic phosphate by pyrophosphatase —and two from GTP—one for binding the
aminoacyl-tRNA to the A site and one for the translocation step (see Figure
31.13). [Note: Additional ATP and GTP molecules are required for initiation in
eukaryotes, whereas an additional GTP molecule is required for termination in
both eukaryotes and prokaryotes.]
Related Topics
