Posttranscriptional Modification of RNA
| Home | | Biochemistry |Chapter: Biochemistry : RNA Structure, Synthesis, and Processing
A primary transcript is the initial, linear, RNA copy of a transcription unit (the segment of DNA between specific initiation and termination sequences).
POSTTRANSCRIPTIONAL MODIFICATION OF RNA
A primary transcript is
the initial, linear, RNA copy of a transcription unit (the segment of DNA
between specific initiation and termination sequences). The primary transcripts
of both prokaryotic and eukaryotic tRNA and rRNA are posttranscriptionally
modified by cleavage of the original transcripts by ribonucleases. tRNAs are
then further modified to help give each species its unique identity. In
contrast, prokaryotic mRNA is generally identical to its primary transcript,
whereas eukaryotic mRNA is extensively modified both co- and
posttranscriptionally.
A. Ribosomal RNA
rRNAs of both
prokaryotic and eukaryotic cells are generated from long precursor molecules
called pre-rRNAs. The 23S, 16S, and 5S rRNA of prokaryotes are produced from a
single pre-rRNA molecule, as are the 28S, 18S, and 5.8S rRNA of eukaryotes
(Figure 30.15). [Note: Eukaryotic 5S rRNA is synthesized by RNA pol III and
modified separately.] The pre-rRNAs are cleaved by ribonucleases to yield
intermediate-sized pieces of rRNA, which are further processed (trimmed by
exonucleases and modified at some bases and riboses) to produce the required
RNA species. [Note: In eukaryotes, rRNA genes are found in long, tandem arrays.
rRNA synthesis and processing occur in the nucleolus, with base and sugar
modifications facilitated by snoRNA. Some of the proteins destined to become
components of the ribosome associate with pre-rRNA prior to and during its
modification.]
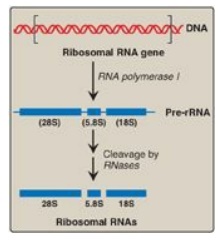
Figure 30.15
Posttranscriptional processing of eukaryotic ribosomal RNA by ribonucleases
(RNases). S = Svedberg unit.
B. Transfer RNA
Both eukaryotic and
prokaryotic tRNAs are also made from longer precursor molecules that must be
modified (Figure 30.16). Sequences at both ends of the molecule are removed,
and, if present, an intron is removed from the anticodon loop by nucleases.
Other posttranscriptional modifications include addition of a –CCA sequence by
nucleotidyltransferase to the 3I -terminal end of tRNA, and modification of
bases at specific positions to produce the “unusual bases” characteristic of
tRNA.
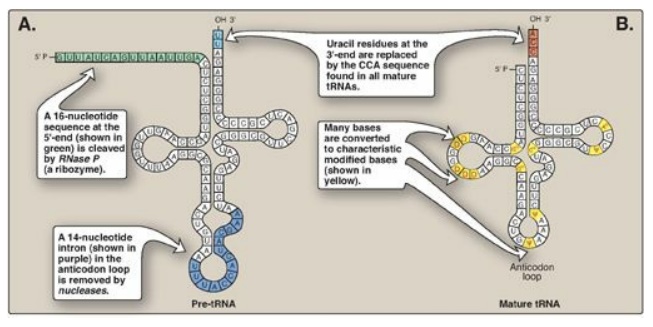
Figure 30.16 A. Primary transfer RNA (tRNA) transcript. B. Functional tRNA after posttranscriptional modification. Modified bases include D (dihydrouracil); Ψ (pseudouracil); and m, which means that the base has been methylated.
C. Eukaryotic mRNA
The collection of all
the primary transcripts synthesized in the nucleus by RNA pol II is known as
heterogeneous nuclear RNA (hnRNA). The pre-mRNA components of hnRNA undergo
extensive co- and posttranscriptional modification in the nucleus. These
modifications usually include the following.
1. 5 “Capping”: This is the first of the processing reactions for pre-mRNA (Figure 30.17). The cap is a 7-methylguanosine attached to the 5I -terminal end of the mRNA through an unusual 5I →5I triphosphate linkage that is resistant to most nucleases. Creation of the cap requires removal of the g phosphoryl group from the 5 - triphosphate of the pre-mRNA, followed by addition of guanosine monophosphate (GMP) (from GTP) by the nuclear enzyme guanylyltransferase. Methylation of this terminal guanine occurs in the cytosol and is catalyzed by guanine-7-methyltransferase. S-adenosylmethionine is the source of the methyl group. Additional methylation steps may occur. The addition of this 7-methylguanosine cap helps stabilize the mRNA and permits efficient initiation of translation.
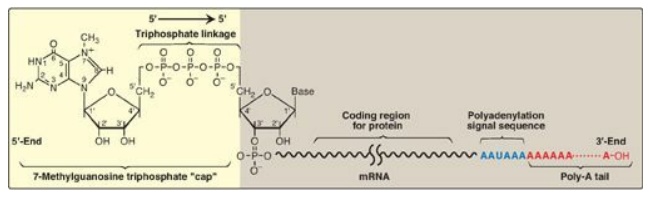
Figure 30.17 Posttranscriptional
modification of messenger RNA (mRNA) showing the 7-methylguanosine cap and
poly-A tail.
2. Addition of a poly-A tail: Most eukaryotic mRNA (with several
notable exceptions, including those coding for the histones) have a chain of
40–250 adenine nucleotides attached to the 3I -end (see Figure 30.17). This
poly-A tail is not transcribed from the DNA, but rather is added after
transcription by the nuclear enzyme, polyadenylate polymerase, using ATP as the
substrate. The pre-mRNA is cleaved downstream of a consensus sequence, called
the polyadenylation signal sequence (AAUAAA), found near the 3I -end of the
RNA, and the poly-A tail is added to the new 3I -end. These tails help
stabilize the mRNA, facilitate its exit from the nucleus, and aid in
translation. After the mRNA enters the cytosol, the poly-A tail is gradually
shortened.
3. Removal of introns: Maturation of eukaryotic mRNA
usually involves removal from the primary transcript of RNA sequences (introns,
or intervening sequences) that do not code for protein. The remaining coding
(expressed) sequences, the exons, are joined together to form the mature mRNA.
The process of removing introns and joining exons is called splicing. The
molecular complex that accomplishes these tasks is known as the spliceosome. A
few eukaryotic primary transcripts contain no introns (for example, those from
histone genes). Others contain a few introns, whereas some, such as the primary
transcripts for the a chains of collagen, contain more than 50 intervening
sequences that must be removed before mature mRNA is ready for translation.
a. Role of small nuclear RNAs: In association with multiple
proteins, uracil-rich snRNAs form small nuclear ribonucleoprotein particles
(snRNPs, or “snurps,” designated as U1, U2, U4, U5, and U6) that mediate
splicing. They facilitate the removal of introns by forming base pairs with the
consensus sequences at each end of the intron (Figure 30.18). [Note: In
systemic lupus erythematosus, an autoimmune disease, patients produce
antibodies against their own nuclear proteins such as snRNPs.]
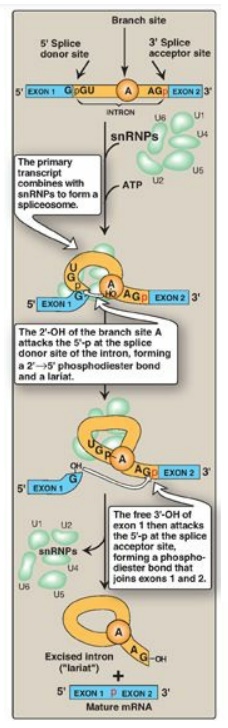
Figure 30.18 Splicing. snRNP = small nuclear ribonucleoprotein particle; mRNA = messenger RNA. [Note: U1 binds the 5I donor site, U2 binds the branch A, and addition of U4-U6 completes the complex.]
b. Mechanism of splicing: The binding of snRNPs brings the
sequences of the neighboring exons into the correct alignment for splicing,
allowing two transesterification reactions to occur. The 2I -OH group of an adenine nucleotide (known as the branch site A)
in the intron attacks the phosphate at the 5I -end of the intron (splice donor
site), forming an unusual 2I →5I phosphodiester bond and creating a “lariat”
structure (see Figure 30.18). The newly freed 3I -OH of exon 1 attacks the 5I
-phosphate at the splice acceptor site, forming a phosphodiester bond that
joins exons 1 and 2. The excised intron is released as a lariat, which is
typically degraded. [Note: The GU and AG sequences at the beginning and end,
respectively, of introns are invariant.] After introns have been removed and
exons joined, the mature mRNA molecules leave the nucleus and pass into the
cytosol through pores in the nuclear membrane. [Note: The introns in tRNA (see
Figure 30.16) are removed by a different mechanism.]
c. Effect of splice site mutations: Mutations at splice sites can lead
to improper splicing and the production of aberrant proteins. It is estimated
that over 15% of all genetic diseases are a result of mutations that affect RNA
splicing. For example, mutations that cause the incorrect splicing of β-globin
mRNA are responsible for some cases of β-thalassemia, a disease in which the production
of the β-globin protein is defective. Splice site mutations can result in exons
being skipped (removed) or introns retained. They can also activate cryptic
splice sites, which are sites that contain the 5 or 3 consensus sequence but
aren’t normally used.
4. Alternative splicing of mRNA molecules: The pre-mRNA molecules from over 50% of human genes can be spliced in alternative ways in different tissues. This produces multiple variations of the mRNA and, therefore, of its protein product (Figure 30.19), and thus is a mechanism for producing a large, diverse set of proteins from a limited set of genes. For example, in eukaryotic cells, the mRNA for tropomyosin, an actin filament–binding protein of the cytoskeleton (and of the contractile apparatus in muscle cells), undergoes extensive tissue-specific alternative splicing with production of multiple isoforms of the tropomyosin protein.
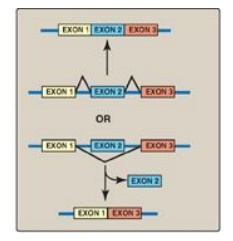
Figure 30.19 Alternative
splicing patterns in eukaryotic messenger RNA.
Related Topics
