Devices
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Inserts, implants, and devices
Devices are specialized pharmaceutical drug delivery systems in which the desired drug delivery and targeting are achieved with the aid of the packag-ing container. Pulmonary delivery devices, transdermal devices, and IUDs exemplify them.
Devices
Devices
are specialized pharmaceutical drug delivery systems in which the desired drug
delivery and targeting are achieved with the aid of the packag-ing container.
Pulmonary delivery devices, transdermal devices, and IUDs exemplify them.
Inhaler devices for pulmonary drug delivery
Delivery
devices play a major role in the efficiency of pulmonary drug delivery. Drug
particles or solution are aerosolized and inhaled with the breath for delivery
to the lung. An aerosol is a colloidal dispersion of a liquid or a solid in a
gas. Aerosol device is a pressurized or breath-actuated dosage forms designed
to deliver the containing solution or suspension of drug(s) to the lung by
forming an aerosol at the time of administration. A pressurized aerosol device
contains a liquid propellant in a pressurizable container, a valve that allows
the pressurized product to be expelled from the container when the actuator is
pressed, and a dip tube that conveys the formulation from the bottom of the
container to the valve assembly. The propellant is a liquefied gas that expands
readily upon release of pressure to provide the driving force for the delivery
of the contents.
Formulation factors affecting pulmonary
drug delivery include par-ticle size and size distribution, shape, and density.
Generally, particles in the size range of 1–5 μm are considered respirable (have
significant lung deposition). Particle shape and density determine the
proportion of inhaled particles that deposit in deep lung alveoli versus major
airways. Device factors affecting pulmonary drug delivery include efficiency of
spray, size and size uniformity of sprayed droplets, location of spray
generation in the context of patient’s anatomy, width of spray zone, and the
speed of the aerosol. User or patient factors impacting drug delivery
to the lung include coordination of inspiration time with device actuation and
the strength, quantity, and consistency of air intake for breath-actuated
devices.
Formulation
considerations important for the development of aerosol dosage forms include uniformity of drug content, especially in the
case of powders and suspension; particle size and size distribution, shape, and
den-sity (for powders and suspensions); flow through the nozzle, compatibility
with the container components, emitted dose, and fine particle dose or fine
particle fraction in the emitted dose.
The
most commonly used devices for pulmonary drug delivery include nebulizers for
liquid formulations, metered-dose inhalers (MDIs), and dry powder inhalers
(DPIs). Figure 24.3 shows schematic of these
inhalation devices. These devices vary as much in their sophistication as they
do in their effectiveness. Each type of device has its own advantages,
disadvan-tages, and limitations. The choice of device depends on the drug (such
as solubility and stability in aqueous medium), the formulation (e.g., dry
pow-der or aqueous solution), pathophysiology of the lungs (e.g., lung
capacity), and the status of the patient (e.g., in patient or ambulatory
self-use).
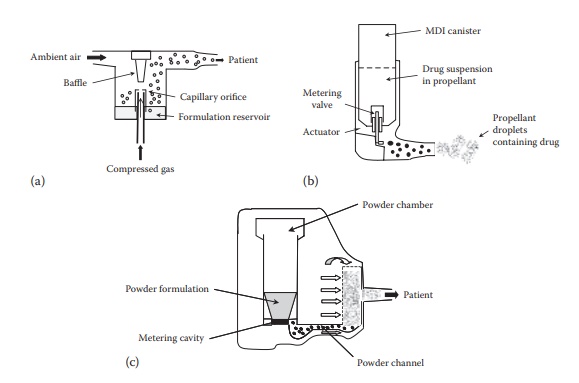
Figure 24.3 An illustration of design elements of inhalation devices: (a)
nebulizer, (b) metered-dose inhaler, and (c) dry powder inhaler.
Nebulizers
Nebulizers
convert aqueous solutions or micronized suspension of a drug into an aerosol
for inhalation using compressed air (usually through a pump) and device design.
The patient inhales normally while the aero-solized product is delivered to the
patient through a mouthpiece adapter. Nebulizers require minimal patient
coordination of breathing but are cum-bersome, nonportable, and time consuming
to use.
There
are two main types of nebulizer:
·
Air-jet (high velocity air stream-aided dispersion)
nebulizers
·
Ultrasonic (ultrasonic energy-aided dispersion) nebulizers
Air-jet
nebulizers rely upon compressed gas
to aerosolize a solution that is then available for inhalation by the patient.
Ultrasonic nebulizers utilize ultrasonic vibrations for aerosolization. Both
air-jet and ultrasonic nebu-lizers produce aerosol at a constant rate
regardless of the respiration cycle. This leads to loss of approximately two
thirds of the aerosol during the expiration and breath-holding phases.
Two
improved nebulizers, the breath-enhanced
nebulizers and dosimetric nebulizers,
overcome this limitation. These inhalers direct the patient’s inhaled air
within the nebulizer to enhance aerosol volume during the inhalation phase and
release aerosol exclusively during the inhalation phase, respectively.
All
nebulizers require a solution or suspension-based formulation, which places
stringent demands on the solubility and stability of the drug in aque-ous
media. For protein and peptide drugs, the stability of proteins and peptides
upon shearing can pose additional limitation. Nebulization exerts high shear
stress on these macromolecules, which can lead to their denatur-ation. This
problem gets exacerbated because 99% of the droplets generated are recycled
back into the reservoir to be nebulized during the next dosing cycle.
Furthermore, the physical properties of drug solutions (e.g., ionic strength,
viscosity, osmolarity, pH, and surface tension) may change with dosing and may
affect the nebulization efficiency. The droplets produced by nebulizers are
heterogeneous in size, which results in very poor drug delivery to the lower
respiratory tract. They often require several minutes of use to administer the
desired dose of medicine. Nebulizers are more effective for drug delivery to
the major pathways of the respiratory track, such as the trachea, than deep
lung alveoli. Thus, adrenergic agonists such as albuterol and steroids such as
budesonide are commonly delivered by nebulizers. Recombinant human DNase,
Dornase alfa (Pulmozyme® by Genentech in South San Francisco, CA) uses
nebulizer for protein delivery to the respiratory tract. Pulmozyme reduces
viscosity of the airway secre-tions by cleaving the extracellular fibrillar
aggregates of DNA from auto-lyzing neutrophils in cystic fibrosis.
New
nebulizer devices, such as the AERx (Aradigm, Hayward, CA) and Respimat
(Boehringer, Germany), generate an aerosol mechanically and reduce the shear
forces on the drug. In addition, vibrating
mesh technolo-gies such as AeroDose (Aerogen Inc., Mountain View, CA) have
been used successfully to deliver proteins to the lungs.
Metered-dose inhalers
MDIs
generate aerosol for inhalation by expelling a measured dose of pres-surized
liquid propellant containing drug via an orifice. They are portable, easy to
use, and the most commonly used inhalation aerosol devices today. A typical MDI
comprises of a canister, metering valve, actuator, spacer, and holding chamber.
In addition, they may also have dose counters and content indicators. During
MDI manufacturing, more aerosol formulation than claimed is commonly added,
which is sufficient for additional 20–30 sprays. However, the last doses from
the container are inconsistent and unpredictable. Therefore, the dose counter
feature allows patients to track the number of actuations and avoids using the
product beyond the recom-mended number of doses.
MDIs
utilize propellants, such as chlorofluorocarbons (CFC) and hydro-fluoroalkanes
(HFAs), to emit the drug solution through a nozzle. A meter-ing chamber within
the valve measures individual doses volumetrically. High velocity of the
generated aerosol spray causes substantial oropharyn-geal deposition by
impaction, which results in poor drug delivery to the lung. This can be avoided
by adding a spacer device, which
reduces aerosol velocity. The spacer also overcomes difficulties in the
coordination of inha-lation and actuation, especially for pediatric patients,
resulting in improved dosing reproducibility.
MDI
delivery efficiency depends on the patient’s inspiratory flow rate, breathing
pattern, and hand–mouth coordination. Increase in tidal volume (volume of air
moved into or out of the lungs during normal breathing) and decrease in
respiratory frequency increase peripheral drug deposition in the lung. Most
patients need to be trained for proper use of the MDI.
Dry powder inhalers
DPIs
are one of the most popular methods of protein delivery to the lungs. DPIs
generate aerosols by drawing air through the loose dry powder of a drug
formulation. These are usually capsule-based devices, wherein the dry powder
formulation is filled and provided in a hard gelatin capsule. The device
pierces the capsule and provides inspiratory air pathway that would fluidize
the capsule and would enable release of its contents through the piercing. The
drug particles form an aerosol in the inspired air upon breath-ing by the
patient. DPIs are generally easier to use, compared to MDIs. However, DPIs
require a rapid rate of inhalation to provide necessary energy for
aerosolization, which may be difficult for pediatric or distressed patients,
and in certain disease states such as asthma or chronic obstructive pulmonary
disease (COPD). DPIs range from unit dose systems, employing only the patient’s
breath to generate the aerosol, to multiple-dosing
reser-voir devices, which actively impart energy to the powder bed to
introduce aerosol particles into the
patient’s respiratory airflow. For stability reasons, unit-dose devices are preferred for protein delivery. Figure 24.3c shows the schematic design of a noncapsule-based DPI (Novolizer®).
Lung
deposition of drug particles varies among different DPIs. DPIs are complex
systems, and their performance depends on effective powder deagglomeration.
Drugs in low doses are often combined with excipients that provide drug-binding
sites on surface while serving as bulking agents. These help with uniformity of
drug content and consistency of emitted dose. Carrier particles, such as
lactose, are commonly added to avoid drug agglomeration due to cohesive forces
among the micronized drug parti-cles. When air is directed through the powder,
turbulent airflow detaches small drug particles from the carrier particles. The
smaller particle size drug migrates to the lung alveoli, whereas the larger
particle size excipient deposits in the back of the throat and in the major
respiratory airways. Thus, optimized performance of both the device and the
formulation is critical to ensuring high and consistent lung deposition.
Most
of the therapeutic dry powders for DPIs are currently made with particles of
small aerodynamic particle diameter (e.g., 90% particles below 5 μm) and density of 1 ± 0.5 g/cm3.
Increased porosity of particles, such as when produced by spray drying, helps
with deep lung penetration by improving the aerodynamic performance. Uniformity
of particle size distri-bution, shape, and density are important for achieving
efficient pulmonary delivery.
Drugs
administered by inhalation are mostly intended to have a direct effect on the
lungs. Inhaled drugs play a very prominent role in the treat-ment of asthma.
This route has significant advantages over oral or paren-teral administration,
because lipid-soluble compounds are rapidly absorbed across the respiratory
tract epithelium. Bronchodilators and corticosteroids are commonly used for
treating asthma and COPD. Azmacort® (triamcino-lone acetamide), Ventolin® HFA
(albuterol sulfate), and Serevent® (salme-terol) are examples of commercially
available aerosols for the treatment of asthma.
Proteins,
oligonucleotides, and genes demonstrate poor oral bioavailabil-ity due to the
harsh environment of the gastrointestinal tract and their hydrophilicity, large
size, and rapid metabolism. In such cases, the pulmo-nary route enables higher
rates of passage into systemic circulation than oral administration.
Intrauterine devices
IUDs,
as the name suggests, are the devices that are placed in the uterus. These
devices are mostly used for contraception by preventing the fertilization of
the egg by the sperm, inhibiting tubular transport, and/or preventing the
implantation of the blastocyst into the uterine endometrium. The hormone
containing devices can be used for other hormonal effects such as in menorrhagia.
IUDs
can be (a) inert, (b) copper based, or (c) hormone containing. Most IUDs are T
shaped so that they are held in place in the uterus by the arms of the T shape. The copper surface of
copper-based IUDs allows the release of copper in the uterine mucosal
microenvironment, which aids contra-ception. A side effect of copper-based IUDs
is increased uterine bleeding. The hormone-based IUDs mostly contain a
progestogen. The use of these devices can provide much lower systemic and high
local progestogen levels.
·
Progestasert® device is designed for implantation into the
uterine cav-ity, where it releases 65 μg progesterone per day to provide
contracep-tion for 1 year.
·
Mirena® device, also known as the LNG-20 IUS (intrauterine
sys-tem), contains levonorgestrel. It is designed to provide an initial drug
release rate of 20 μg/day
and is used to provide contraception for up to 5 years.
Subcutaneous devices
Parenteral
drug administration is indicated for several drugs, especially the new
biotechnology-based drug products such as monoclonal antibod-ies, and protein
and peptide therapeutic agents. During the initial clinical development of
these therapies, IV route of drug administration is adopted to allow dose
flexibility and the ability to closely monitor and control drug exposure.
However, the IV route of drug administration is not preferred for commercial
use of these drugs by patients because IV drug adminis-tration requires the
intervention of a health care professional and the use of a health care
facility. In contrast, SC administration is often preferred because it can
allow for patient self-administration at home and utilizes smaller size needle,
which causes less pain at the injection site and is more patient friendly. The
development of SC dosage forms of drugs has, there-fore, gathered significant
momentum in the recent years.
At
the same time, SC delivery of several drugs is limited by the injection volume
that can be administered in the SC space (usually about 1 mL, maximum 1.5 mL),
the required dose of the drug, and the solubility of the drug in the injection
vehicle (for a soluble drug product). Based on the dose and the solubility
calculations, the required dosing volume of a drug can sometimes exceed the
usual injectable volume in the SC space. In these cases, the use of a device
for SC drug delivery can facilitate SC delivery of a drug that would otherwise
not be possible.
The
devices that can be used for SC delivery of a drug could be a syringe pump in
an inpatient setting or a patch pump in an outpatient setting.
A
syringe pump is a mechanical device that pumps the drug product through a
syringe at a low enough rate to match the absorption of the fluid from the
injected SC space. This allows higher volume of drug to be administered
subcutaneously over a prolonged period of time. The use of a syringe pump,
however, requires the patient site of drug administration to be immobilized for
the duration of drug administration. Also, the operation and calibration of the
syringe pump usually require the expertise of a health care profes-sional.
Thus, although syringe pumps can generally be used in an inpatient setting in a
hospital and during clinical trials, their use is limited for out-patient
clinical use.
SC
devices for outpatient clinical use are generally smaller, battery-operated
pumps that can be attached to the abdominal cavity and worn under the clothing.
These allow patient self-administration and the patient can have mobility
during drug administration. Such devices are exemplified by Roche’s MyDose®
device and West’s SmartDose® electronic wearable injector. The improved quality
of life and patient convenience associated with these devices provides better
patient compliance and satisfaction with the therapy.
Related Topics
