Enzymic Changes in the Absorptive State
| Home | | Biochemistry |Chapter: Biochemistry : The Feed-Fast Cycle
The flow of intermediates through metabolic pathways is controlled by four mechanisms: 1) the availability of substrates; 2) allosteric regulation of enzymes; 3) covalent modification of enzymes; and 4) induction-repression of enzyme synthesis, primarily through regulation of transcription.
ENZYMIC CHANGES IN THE ABSORPTIVE STATE
The flow of
intermediates through metabolic pathways is controlled by four mechanisms: 1)
the availability of substrates; 2) allosteric regulation of enzymes; 3)
covalent modification of enzymes; and 4) induction-repression of enzyme
synthesis, primarily through regulation of transcription. Although this scheme
may at first seem redundant, each mechanism operates on a different timescale
(Figure 24.1) and allows the body to adapt to a wide variety of physiologic
situations. In the well-fed state, these regulatory mechanisms ensure that
available nutrients are captured as glycogen, TAG, and protein.
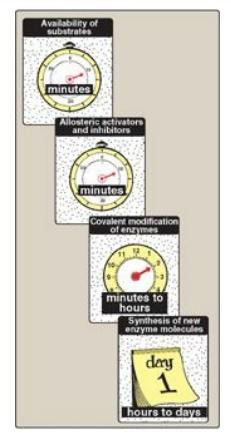
Figure 24.1 Control mechanisms of metabolism and some typical response times. [Note: Response times may vary according to the nature of the stimulus and from tissue to tissue.]
A. Allosteric effectors
Allosteric changes
usually involve rate-determining reactions. For example, glycolysis in the
liver is stimulated following a meal by an increase in fructose
2,6-bisphosphate, an allosteric activator of phosphofructokinase-1 ([PFK-1]).
In contrast, gluconeogenesis is inhibited by fructose 2,6-bisphosphate, an
allosteric inhibitor of fructose 1,6-bisphosphatase.
B. Covalent modification
The activity of many
enzymes is regulated by the addition (via kinases, such as cyclic adenosine
monophosphate [cAMP]-activated protein kinase A [PKA] and adenosine
monophosphate-activated protein kinase [AMPK]) or removal (via phosphatases) of
phosphate groups from specific serine, threonine, or tyrosine residues of the
protein. In the absorptive state, most of the covalently regulated enzymes are
in the dephosphorylated form and are active (Figure 24.2). Three exceptions are
glycogen phosphorylase kinase, glycogen phosphorylase, and hormone-sensitive
lipase (HSL) of adipose tissue, which are inactive in their dephosphorylated
form. [Note: In liver, the phosphatase domain of bifunctional
phosphofructokinase-2 (PFK-2) is inactive when the protein is
dephosphorylated.]
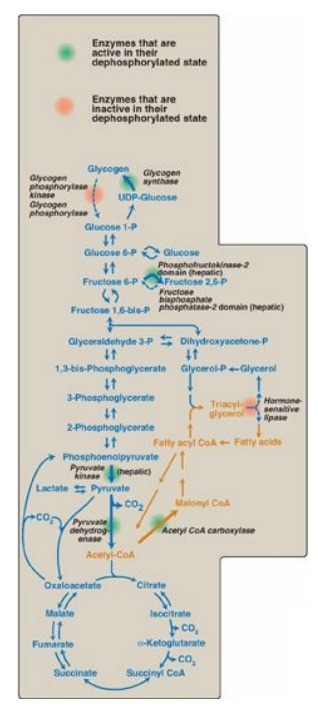
Figure 24.2 Important reactions of intermediary metabolism regulated by enzyme phosphorylation. Blue text = intermediates of carbohydrate metabolism; brown text = intermediates of lipid metabolism. P = phosphate; CoA = coenzyme A.
C. Induction and repression of enzyme synthesis
Increased (induction of) or decreased (repression of) enzyme synthesis leads to changes in the number of enzyme molecules, rather than influencing the activity of existing enzyme molecules. Enzymes subject to regulation of synthesis are often those that are needed under specific physiologic conditions. For example, in the fed state, elevated insulin levels result in an increase in the synthesis of key enzymes, such as acetyl coenzyme A (CoA) carboxylase ([ACC]) and fatty acid synthase, involved in anabolic metabolism. In the fasted state, glucagon induces expression of phosphoenolpyruvate carboxykinase (PEPCK) of gluconeogenesis. Both hormones affect transcription factors.
Related Topics
