Liver: Nutrient Distribution Center
| Home | | Biochemistry |Chapter: Biochemistry : The Feed-Fast Cycle
The liver is uniquely situated to process and distribute dietary nutrients because the venous drainage of the gut and pancreas passes through the hepatic portal vein before entry into the general circulation.
LIVER: NUTRIENT DISTRIBUTION CENTER
The liver is uniquely situated
to process and distribute dietary nutrients because the venous drainage of the
gut and pancreas passes through the hepatic portal vein before entry into the
general circulation. Thus, after a meal, the liver is bathed in blood
containing absorbed nutrients and elevated levels of insulin secreted by the
pancreas. During the absorptive period, the liver takes up carbohydrates,
lipids, and most amino acids. These nutrients are then metabolized, stored, or
routed to other tissues. In this way, the liver smooths out potentially broad
fluctuations in the availability of nutrients for the peripheral tissues.
A. Carbohydrate metabolism
Liver is normally a
glucose-producing rather than a glucose-using tissue. However, after a meal
containing carbohydrate, the liver becomes a net consumer, retaining roughly 60
of every 100 g of glucose presented by the portal system. This increased use
reflects increased glucose uptake by the hepatocytes. Their insulin-independent
glucose transporter (GLUT-2) has a low affinity (high K m) for glucose and,
therefore, takes up glucose only when blood glucose is high. Additional
mechanisms by which hepatic glucose metabolism is increased include the
following. [Note: The numbers in colored circles in the text refer to Figure
24.3.]
1. Increased phosphorylation of glucose: The elevated levels of glucose
within the hepatocyte (as a result of elevated extracellular levels) allow
glucokinase to phosphorylate glucose to glucose 6-phosphate (Figure 24.3,1).
(Recall that glucokinase has a high Km for glucose, is not subject to direct
product inhibition, and has a sigmoidal reaction curve;).
2. Increased glycogenesis: The conversion of glucose
6-phosphate to glycogen is favored by the activation of glycogen synthase, both
by dephosphorylation and by increased availability of glucose 6-phosphate, its
allosteric effector (see Figure 24.3,2).
3. Increased activity of the pentose phosphate
pathway: The
increased availability of glucose 6-phosphate, combined with the active use of
nicotinamide adenine dinucleotide phosphate (NADPH) in hepatic lipogenesis,
stimulates the pentose phosphate pathway ([PPP]). This pathway typically
accounts for 5%–10% of the glucose metabolized by the liver (see Figure 24.3,3).
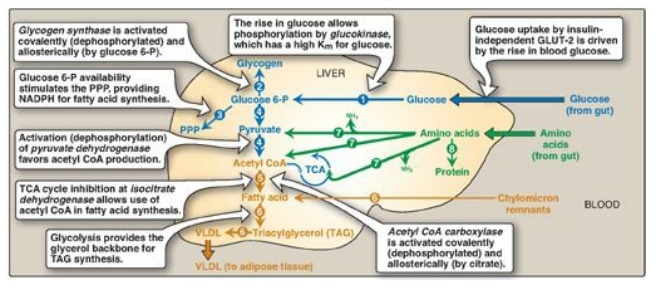
Figure 24.3 Major metabolic
pathways in liver in the absorptive state. [Note: The acetyl CoA is also used
for cholesterol synthesis.] The numbers in circles, which appear both in the
figure and in the text, indicate important pathways for carbohydrate, fat, or
protein metabolism. Blue text
= intermediates of carbohydrate metabolism; brown
text = intermediates of lipid metabolism; green text = intermediates of protein
metabolism. P = phosphate; PPP = pentose phosphate pathway; TCA = tricarboxylic
acid (cycle); CoA = coenzyme A; VLDL = very-low-density lipoprotein; GLUT =
glucose transporter; NADPH = nicotinamide adenine dinucleotide phosphate.
4. Increased glycolysis: In liver, glycolysis is
significant only during the absorptive period following a carbohydrate-rich
meal. The conversion of glucose to pyruvate is stimulated by the elevated
insulin-to-glucagon ratio that results in increased amounts of the regulated
enzymes of glycolysis: glucokinase, PFK-1, and pyruvate kinase ([PK]).
Additionally, PFK-1 is allosterically activated by fructose 2,6-bisphosphate
generated by the active (dephosphorylated) kinase domain of bifunctional PFK-2.
PK is dephosphorylated and active. Pyruvate dehydrogenase (PDH), which converts
pyruvate to acetyl CoA, is active (dephosphorylated) because pyruvate inhibits
PDH kinase (see Figure 24.3). The acetyl CoA either is used as a substrate for
fatty acid (FA) synthesis or is oxidized for energy in the tricarboxylic acid
(TCA) cycle. (See Figure 24.4 for the central role of glucose 6-phosphate.)
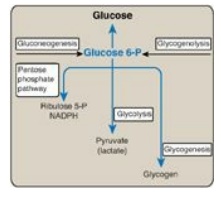
Figure 24.4 Central role of glucose 6-phosphate in metabolism. [Note: The presence of glucose 6-phosphatase in liver allows the production of free glucose from glycogenolysis and gluconeogenesis.] NADPH = nicotinamide adenine dinucleotide phosphate; P = phosphate.
5. Decreased production of glucose: Although glycolysis and glycogenesis (pathways that promote glucose storage) are stimulated in liver in the absorptive state, gluconeogenesis and glycogenolysis (pathways that generate glucose) are decreased. Pyruvate carboxylase (PC), which catalyzes the first step in gluconeogenesis, is largely inactive due to low levels of acetyl CoA, its allosteric activator. [Note: The acetyl CoA is being used for fatty acid synthesis.] The high insulin-to-glucagon ratio also favors inactivation of other gluconeogenic enzymes such as fructose 1,6-bisphosphatase (see Figure 8.17). Glycogenolysis is inhibited by dephosphorylation of glycogen phosphorylase and phosphorylase kinase.
B. Fat metabolism
1. Increased fatty acid synthesis: Liver is the primary tissue for de
novo synthesis of FAs (see Figure 24.3, 5). FA synthesis, a cytosolic process,
is favored in the absorptive period by availability of the substrates acetyl
CoA (from glucose and amino acid metabolism) and NADPH (from glucose
metabolism) and by the activation of ACC, both by dephosphorylation and by the
presence of its allosteric activator, citrate. [Note: Inactivity of AMPK favors
dephosphorylation.] ACC catalyzes the formation of malonyl CoA from acetyl CoA,
the rate-limiting reaction for FA synthesis. [Note: Malonyl CoA inhibits
carnitine palmitoyltransferase-I (CPT-I) of FA oxidation. Citrate, thereby,
directly activates FA synthesis and indirectly inhibits FA degradation.]
a. Source of cytosolic acetyl coenzyme A: Pyruvate from aerobic glycolysis
enters mitochondria and is decarboxylated by PDH. The acetyl CoA product is
combined with oxaloacetate (OAA) to form citrate via citrate synthase. Citrate
leaves the mitochondria (as a result of the inhibition of isocitrate
dehydrogenase by adenosine triphosphate [ATP]) and enters the cytosol. Citrate
is cleaved by ATP-citrate lyase (induced by insulin), producing the acetyl CoA
substrate of ACC and OAA. The OAA is reduced to malate, which is oxidatively decarboxylated
to pyruvate by malic enzyme as NADPH is formed.
2. Increased triacylglycerol synthesis: TAG synthesis is favored because fatty acyl CoAs are available both from de novo synthesis from acetyl CoA and from hydrolysis of the TAG component of chylomicron remnants removed from the blood by hepatocytes. Glycerol 3-phosphate, the backbone for TAG synthesis, is provided by glycolysis. The liver packages TAG into very-low-density lipoprotein (VLDL) particles that are secreted into the blood for use by extrahepatic tissues, particularly adipose and muscle tissues (see Figure 24.3, 6).
C. Amino acid metabolism
1. Increased amino acid degradation: In the absorptive period, more
amino acids are present than the liver can use in the synthesis of proteins and
other nitrogen-containing molecules. The surplus amino acids are not stored but
are either released into the blood for other tissues to use in protein
synthesis or deaminated, with the resulting carbon skeletons being degraded by
the liver to pyruvate, acetyl CoA, or TCA cycle intermediates. These
metabolites can be oxidized for energy or used in FA synthesis (see Figure
24.3, 7). The liver has limited capacity to degrade the branched-chain amino
acids (BCAAs) leucine, isoleucine, and valine. They pass through the liver
essentially unchanged and are preferentially metabolized in muscle.
2. Increased protein synthesis: The body does not store protein in
the same way that it maintains glycogen or TAG reserves. However, a transient
increase in the synthesis of hepatic proteins does occur in the absorptive
state, resulting in replacement of any proteins that may have been degraded
during the previous postabsorptive period (see Figure 24.3, 8).
Related Topics
