Glucagon
| Home | | Biochemistry |Chapter: Biochemistry : Metabolic Effects of Insulin and Glucagon
Glucagon is a peptide hormone secreted by the α cells of the pancreatic islets of Langerhans.
GLUCAGON
Glucagon is a peptide
hormone secreted by the α cells of the pancreatic islets of Langerhans.
Glucagon, along with epinephrine, norepinephrine, cortisol, and growth hormone
(the “counterregulatory” hormones), opposes many of the actions of insulin
(Figure 23.10). Most importantly, glucagon acts to maintain blood glucose
levels by activation of hepatic glycogenolysis and gluconeogenesis. Glucagon is
composed of 29 amino acids arranged in a single polypeptide chain. [Note:
Unlike insulin, the amino acid sequence of glucagon is the same in all
mammalian species examined to date.] Glucagon is synthesized as a large
precursor molecule (preproglucagon) that is converted to glucagon through a
series of selective proteolytic cleavages, similar to those described for
insulin biosynthesis (see Figure 23.3). In contrast to insulin, preproglucagon
is processed to different products in different tissues, for example, GLP-1 in
intestinal L cells. Like insulin, glucagon has a short half-life.

Figure 23.10 Opposing actions
of insulin and glucagon plus epinephrine.
A. Stimulation of glucagon secretion
The α cell is
responsive to a variety of stimuli that signal actual or potential hypoglycemia
(Figure 23.11). Specifically, glucagon secretion is increased by low blood
glucose, amino acids, and catecholamines.
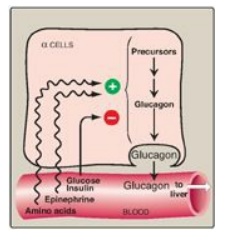
Figure 23.11 Regulation of glucagon release from pancreatic α cells. [Note: Amino acids increase release of insulin and glucagon, whereas glucose increases release of insulin only.]
1. Low blood glucose: A decrease in plasma glucose concentration is the primary stimulus for glucagon release. During an overnight or prolonged fast, elevated glucagon levels prevent hypoglycemia (see below for a discussion of hypoglycemia).
2. Amino acids: Amino acids (for example, arginine) derived from a
meal containing protein stimulate the release of glucagon. The glucagon
effectively prevents the hypoglycemia that would otherwise occur as a result of
the increased insulin secretion that also occurs after a protein meal.
3. Catecholamines: Elevated levels of circulating
epinephrine produced by the adrenal medulla, norepinephrine produced by
sympathetic innervation of the pancreas, or both stimulate the release of
glucagon. Thus, during periods of physiologic stress, the elevated
catecholamine levels can override the effect on the α cell of circulating
substrates. In these situations, regardless of the concentration of blood
glucose, glucagon levels are elevated in anticipation of increased glucose use.
In contrast, insulin levels are depressed.
B. Inhibition of glucagon secretion
Glucagon secretion is
significantly decreased by elevated blood glucose and by insulin. Both
substances are increased following ingestion of glucose or a carbohydrate-rich
meal (see Figure 23.5). The regulation of glucagon secretion is summarized in
Figure 23.11.
C. Metabolic effects of glucagon
Glucagon is a catabolic
hormone that promotes the maintenance of blood glucose levels. Its primary
target is the liver.
1. Effects on carbohydrate metabolism: The IV administration of glucagon
leads to an immediate rise in blood glucose. This results from an increase in
the breakdown of liver glycogen and an increase in hepatic gluconeogenesis.
2. Effects on lipid metabolism: The primary effect of glucagon on
lipid metabolism is inhibition of fatty acid synthesis through phosphorylation
of ACC. The decrease in malonyl CoA production as a result of ACC inhibition
removes the break on long-chain fatty acid β-oxidation. Glucagon also plays a
role in lipolysis in adipose, but the major activators of hormone sensitive
lipase (via phosphorylation by protein kinase A) are the catecholamines. The
free fatty acids released are taken up by liver and oxidized to acetyl CoA,
which is used in ketone body synthesis.
3. Effects on protein metabolism: Glucagon increases uptake by the liver of amino acids supplied by muscle, resulting in increased availability of carbon skeletons for gluconeogenesis. As a consequence, plasma levels of amino acids are decreased.
D. Mechanism of action of glucagon
Glucagon binds to
high-affinity G protein–coupled receptors on the cell membrane of hepatocytes.
The receptors for glucagon are distinct from those that bind insulin or
epinephrine. [Note: Glucagon receptors are not found on skeletal muscle.]
Glucagon binding results in activation of adenylyl cyclase in the plasma
membrane (Figure 23.12;). This causes a rise in cyclic adenosine monophosphate
(cAMP), which, in turn, activates cAMP-dependent protein kinase A and increases
the phosphorylation of specific enzymes or other proteins. This cascade of increasing
enzymic activities results in the phosphorylation-mediated activation or
inhibition of key regulatory enzymes involved in carbohydrate and lipid
metabolism. An example of such a cascade in glycogen degradation is shown in
Figure 11.9. [Note: Glucagon, like insulin, affects gene transcription. For
example, glucagon induces expression of phosphoenolpyruvate carboxykinase.]
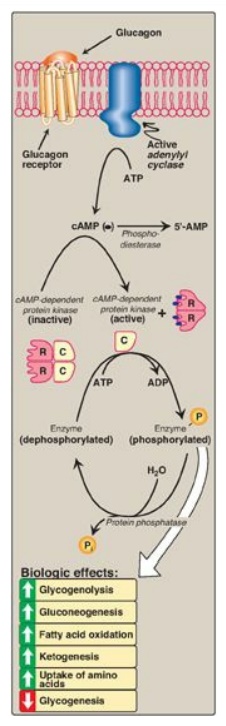
Figure 23.12 Mechanism of action of glucagon. [Note: For clarity, G-protein activation of adenylyl cyclase has been omitted.] R = regulatory subunit; C = catalytic subunit; cAMP = cyclic adenosine monophosphate; ADP = adenosine diphosphate; P = phosphate.
Related Topics
