Pyrimidine Synthesis and Degradation
| Home | | Biochemistry |Chapter: Biochemistry : Nucleotide Metabolism
Unlike the synthesis of the purine ring, which is constructed on a preexisting ribose 5-phosphate, the pyrimidine ring is synthesized before being attached to ribose 5-phosphate, which is donated by PRPP.
PYRIMIDINE SYNTHESIS AND DEGRADATION
Unlike the synthesis of
the purine ring, which is constructed on a preexisting ribose 5-phosphate, the
pyrimidine ring is synthesized before being attached to ribose 5-phosphate,
which is donated by PRPP. The sources of the atoms in the pyrimidine ring are
glutamine, CO2, and aspartate (Figure 22.19).
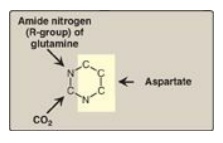
Figure 22.19 Sources of the
individual atoms in the pyrimidine ring.
A. Synthesis of carbamoyl phosphate
The regulated step of
this pathway in mammalian cells is the synthesis of carbamoyl phosphate from
glutamine and CO2, catalyzed by carbamoyl phosphate synthetase (CPS)
II. CPS II is inhibited by uridine triphosphate (the end product of this
pathway, which can be converted into the other pyrimidine nucleotides), and is
activated by PRPP. [Note: Carbamoyl phosphate, synthesized by CPS I, is also a
precursor of urea. Defects in ornithine transcarbamylase of the urea cycle
promote pyrimidine synthesis due to increased availability of carbamoyl
phosphate. A comparison of the two enzymes is presented in Figure 22.20.]
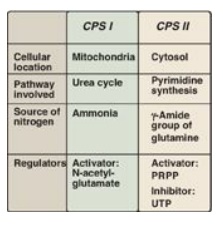
Figure 22.20 Summary of the
differences between carbamoyl phosphate synthetase (CPS) I and II. PRPP =
5-phosphoribosyl-1-pyrophosphate; UTP = uridine triphosphate.
B. Synthesis of orotic acid
The second step in
pyrimidine synthesis is the formation of carbamoylaspartate, catalyzed by
aspartate transcarbamoylase. The pyrimidine ring is then closed by
dihydroorotase. The resulting dihydroorotate is oxidized to produce orotic acid
(orotate) as shown in Figure 22.21. The enzyme that produces orotate,
dihydroorotate dehydrogenase, is a flavoprotein associated with the inner
mitochondrial membrane. All other enzymes in pyrimidine biosynthesis are cytosolic.
[Note: The first three enzymic activities in this pathway (CPS II, aspartate
transcarbamoylase, and dihydroorotase) are actually three different catalytic
domains of a single polypeptide known as CAD from the first letter in the name
of each domain. This is an example of a multfunctional or multicatalytic
polypeptide that facilitates the ordered synthesis of an important compound.
Synthesis of the purine nucleotide IMP also involves multifunctional proteins.]
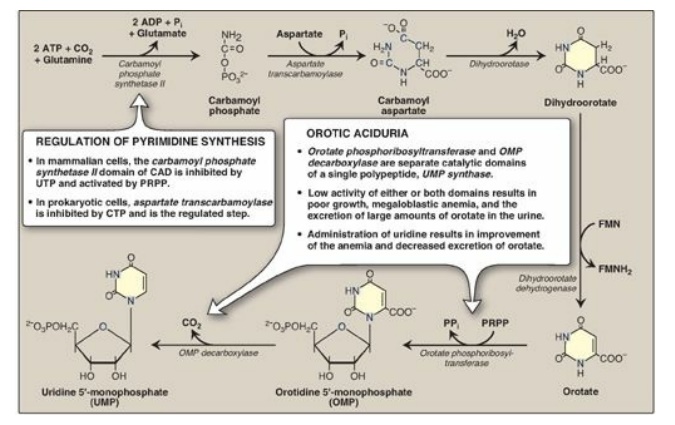
Figure 22.21 De novo pyrimidine synthesis. ADP = adenosine diphosphate; Pi = inorganic phosphate; FMN(H2) = flavin mononucleotide; CTP = cytidine triphosphate; PRPP = 5-phosphoribosyl-1-pyrophosphate; PPi = pyrophosphate.
C. Formation of a pyrimidine nucleotide
The completed pyrimidine ring is converted to the nucleotide orotidine monophosphate (OMP) in the second stage of pyrimidine nucleotide synthesis (see Figure 22.21). PRPP is again the ribose 5-phosphate donor. The enzyme orotate phosphoribosyltransferase produces OMP and releases pyrophosphate, thereby making the reaction biologically irreversible. [Note: Both purine and pyrimidine synthesis require glutamine, aspartic acid, and PRPP as essential precursors.] OMP, the parent pyrimidine mononucleotide, is converted to uridine monophosphate (UMP) by orotidylate decarboxylase, which removes the carboxyl group. Orotate phosphoribosyltransferase and orotidylate decarboxylase are also catalytic domains of a single polypeptide chain called UMP synthase. Orotic aciduria (a very rare disorder) may be caused by a deficiency of one or both activities of this bifunctional enzyme, resulting in orotic acid in the urine (see Figure 22.21). UMP is sequentially phosphorylated to UDP and UTP. [Note: The UDP is a substrate for ribonucleotide reductase, which generates dUDP. The dUDP is phosphorylated to dUTP, which is rapidly hydrolyzed to dUMP by UTP diphosphatase (dUTPase). dUTPase, then, plays an important role in reducing availability of dUTP as a substrate for DNA synthesis, thereby preventing erroneous incorporation of uracil into DNA.]
D. Synthesis of cytidine triphosphate
Cytidine triphosphate
(CTP) is produced by amination of UTP by CTP synthetase (Figure 22.22), with
glutamine providing the nitrogen. [Note: Some CTP is dephosphorylated to
cytidine diphosphate (CDP), which is a substrate for ribonucleotide reductase.
The dCDP product can be phosphorylated to dCTP for DNA synthesis or
dephosphoprylated to dCMP that is deaminated to dUMP.]
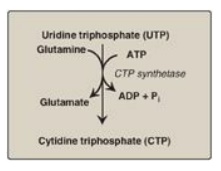
Figure 22.22 Synthesis of CTP
from UTP. [Note: CTP, required for RNA synthesis, is converted to dCTP for DNA
synthesis.] ADP = adenosine diphosphate; Pi = inorganic phosphate.
E. Synthesis of deoxythymidine monophosphate
dUMP is converted to
deoxythymidine monophosphate (dTMP) by thymidylate synthase, which uses
N5,N10-methylene tetrahydrofolate as the source of the methyl group. This is an
unusual reaction in that tetrahydrofolate (THF) contributes not only a
one-carbon unit but also two hydrogen atoms from the pteridine ring, resulting
in the oxidation of THF to dihydrofolate ([DHF] Figure 22.23). Inhibitors of
thymidylate synthase include thymine analogs such as 5-fluorouracil, which
serve as antitumor agents. 5-Fluorouracil is metabolically converted to
5-fluorodeoxyuridine monophosphate (5-FdUMP), which becomes permanently bound
to the inactivated thymidylate synthase, making the drug a “suicide” inhibitor.
DHF can be reduced to THF by dihydrofolate reductase (see Figure 28.3), an
enzyme that is inhibited by folate analogs such as methotrexate. By decreasing
the supply of THF, these drugs not only inhibit purine synthesis (see Figure
22.7), but, by preventing methylation of dUMP to dTMP, they also decrease the
availability of this essential component of DNA. DNA synthesis is inhibited and
cell growth slowed. Thus, these drugs are used to decrease the growth rate of
cancer cells. [Note: Acyclovir (a purine analog) and AZT (3 I -azido-3 I - deoxythymidine, a pyrimidine analog) are used to treat
infections of herpes simplex virus and human immunodeficiency virus,
respectively. Each inhibits the viral DNA polymerase.]
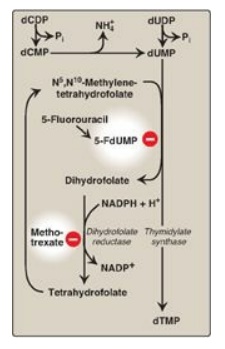
Figure 22.23 Synthesis of dTMP
from dUMP, illustrating sites of action of antineoplastic drugs.
F. Degradation and salvage of pyrimidines
Unlike the purine ring,
which is not cleaved in humans, the pyrimidine ring is opened and degraded to
highly soluble products, β-alanine (from the degradation of CMP and UMP) and
β-aminoisobutyrate (from TMP degradation), with the production of NH3
and CO2. Pyrimidine bases can be salvaged to nucleosides, which are
phosphorylated to nucleotides. However, their high solubility makes pyrimidine
salvage less significant clinically than purine salvage. [Note: The salvage of
pyrimidine nucleosides is the basis for using uridine in the treatment of
hereditary orotic aciduria.]
Related Topics
