Synthesis of Purine Nucleotides
| Home | | Biochemistry |Chapter: Biochemistry : Nucleotide Metabolism
The purine ring is constructed primarily in the liver by a series of reactions that add the donated carbons and nitrogens to a preformed ribose 5-phosphate.
SYNTHESIS OF PURINE NUCLEOTIDES
The atoms of the purine
ring are contributed by a number of compounds, including amino acids
(aspartate, glycine, and glutamine), CO2, and N10-formyltetrahydrofolate
(Figure 22.5). The purine ring is constructed primarily in the liver by a
series of reactions that add the donated carbons and nitrogens to a preformed
ribose 5-phosphate.
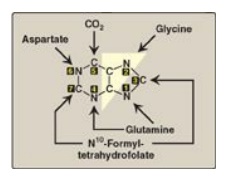
Figure 22.5 Sources of the individual atoms in the purine ring. The order in which the atoms are added is shown by the numbers in the black boxes (see Figure 22.7).
A. Synthesis of 5-phosphoribosyl-1-pyrophosphate
5-Phosphoribosyl-1-pyrophosphate
(PRPP) is an “activated pentose” that participates in the synthesis and salvage
of purines and pyrimidines. Synthesis of PRPP from ATP and ribose 5-phosphate
is catalyzed by PRPP synthetase (Figure 22.6). This X-linked enzyme is
activated by inorganic phosphate and inhibited by purine nucleotides
(end-product inhibition). [Note: The sugar moiety of PRPP is ribose, and,
therefore, ribonucleotides are the end products of de novo purine synthesis.
When deoxyribonucleotides are required for DNA synthesis, the ribose sugar
moiety is reduced.]
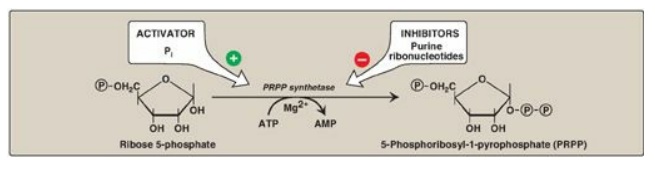
Figure 22.6 Synthesis of PRPP, showing the activator and inhibitors of the reaction. [Note: This is not the committed step of purine synthesis because PRPP is used in other pathways.] P= phosphate; Pi = inorganic phosphate; AMP = adenosine monophosphate.
B. Synthesis of 5-phosphoribosylamine
Synthesis of 5-phosphoribosylamine from PRPP and glutamine is shown in Figure 22.7. The amide group of glutamine replaces the pyrophosphate group attached to carbon 1 of PRPP. This is the committed step in purine nucleotide biosynthesis. The enzyme, glu-tamine:phosphoribosylpyrophosphate amidotransferase, is inhibited by the purine 5 -nucleotides AMP and guanosine monophosphate ([GMP] also called guanylate), the end products of the pathway. The rate of the reaction is also controlled by the intracellular concentration of PRPP. [Note: The concentration of PRPP is normally far below the Michaelis constant (Km) for the amidotransferase. Therefore, any small change in the PRPP concentration causes a proportional change in rate of the reaction.]
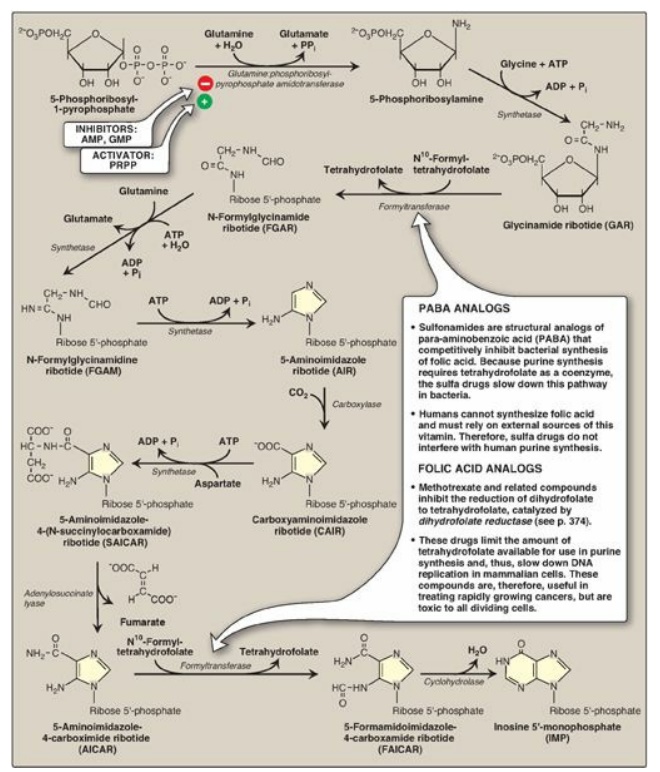
Figure 22.7 De novo synthesis of purine nucleotides, showing the inhibitory effect of some structural analogs. AMP = adenosine monophosphate; ADP = adenosine diphosphate; GMP = guanosine monophosphate; PRPP = 5-phosphoribosyl- 1-pyrophosphate; Pi = inorganic phosphate; PPi = pyrophosphate.
C. Synthesis of inosine monophosphate, the “parent” purine nucleotide
The next nine steps in purine nucleotide biosynthesis leading to the synthesis of inosine monophosphate ([IMP] whose base is hypoxanthine) are illustrated in Figure 22.7. Four steps in this pathway require ATP as an energy source, and two steps in the pathway require N10-formyltetrahydrofolate as a one-carbon donor. [Note: Hypoxanthine is found in tRNA.]
D. Synthetic inhibitors of purine synthesis
Some synthetic inhibitors of purine synthesis (for example, the sulfonamides), are designed to inhibit the growth of rapidly dividing microorganisms without interfering with human cell functions (see Figure 22.7). Other purine synthesis inhibitors, such as structural analogs of folic acid (for example, methotrexate), are used pharmacologically to control the spread of cancer by interfering with the synthesis of nucleotides and, therefore, of DNA and RNA (see Figure 22.7).
Inhibitors of human purine synthesis are extremely
toxic to tissues, especially to developing structures such as in a fetus, or to
cell types that normally replicate rapidly, including those of bone marrow,
skin, gastrointestinal (GI) tract, immune system, or hair follicles. As a
result, individuals taking such anticancer drugs can experience adverse
effects, including anemia, scaly skin, GI tract disturbance,
immunodeficiencies, and hair loss.
E. Synthesis of adenosine and guanosine monophosphate
The conversion of IMP
to either AMP or GMP uses a two-step, energy-requiring pathway (Figure 22.8).
Note that the synthesis of AMP requires guanosine triphosphate (GTP) as an
energy source, whereas the synthesis of GMP requires ATP. Also, the first
reaction in each pathway is inhibited by the end product of that pathway. This
provides a mechanism for diverting IMP to the synthesis of the purine present
in lesser amounts. If both AMP and GMP are present in adequate amounts, the de
novo pathway of purine synthesis is turned off at the amidotransferase step.
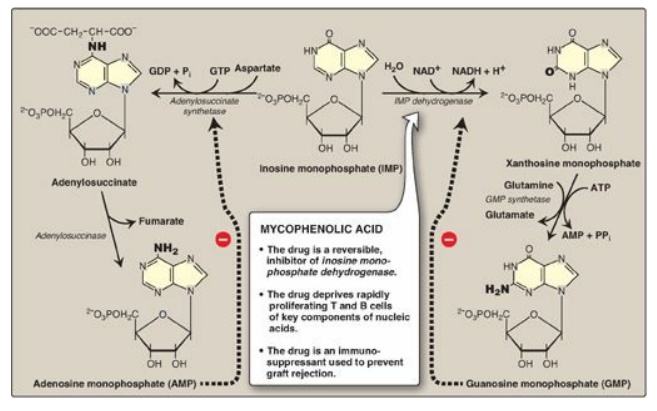
Figure 22.8 Conversion of IMP
to AMP and GMP showing feedback inhibition. [Note: AMP is also called
adenylate. GMP is also called guanylate.] NAD(H) = nicotinamide adenine
dinucleotide; GDP = guanosine diphosphate; GTP = guanosine triphosphate; AMP =
adenosine monophosphate; Pi = inorganic phosphate; PPi = pyrophosphate.
F. Conversion of nucleoside monophosphates to nucleoside diphosphates and triphosphates
Nucleoside diphosphates
are synthesized from the corresponding nucleoside monophosphates by
base-specific nucleoside monophosphate kinases (Figure 22.9). [Note: These
kinases do not discriminate between ribose or deoxyribose in the substrate.]
ATP is generally the source of the transferred phosphate because it is present
in higher concentrations than the other nucleoside triphosphates. Adenylate
kinase is particularly active in the liver and in muscle, where the turnover of
energy from ATP is high. Its function is to maintain equilibrium among the
adenine nucleotides (AMP, ADP, and ATP). Nucleoside diphosphates and triphosphates
are interconverted by nucleoside diphosphate kinase, an enzyme that, unlike the
monophosphate kinases, has broad substrate specificity.
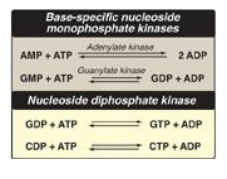
Figure 22.9 Conversion of
nucleoside monophosphates to nucleoside diphosphates and triphosphates.
AMP = adenosine monophosphate;
ADP = adenosine diphosphate;
GMP = guanosine monophosphate;
GDP = guanosine diphosphate;
GTP = guanosine triphosphate;
CDP = cytidine diphosphate;
CTP = cytidine triphosphate.
G. Salvage pathway for purines
Purines that result
from the normal turnover of cellular nucleic acids, or the small amount that is
obtained from the diet and not degraded, can be converted to nucleoside
triphosphates and used by the body. This is referred to as the “salvage
pathway” for purines. [Note: Salvage is particularly important in the brain.]
1. Salvage of purine bases to nucleotides: Two enzymes are involved: adenine phosphoribosyltransferase
(APRT) and hypoxanthine-guanine phosphoribosyltransferase (HGPRT). Both enzymes
use PRPP as the source of the ribose 5-phosphate group (Figure 22.10). The
release of pyrophosphate and its subsequent hydrolysis by pyrophosphatase makes
these reactions irreversible. [Note: Adenosine is the only purine nucleoside to
be salvaged. It is phosphorylated to AMP by adenosine kinase.]
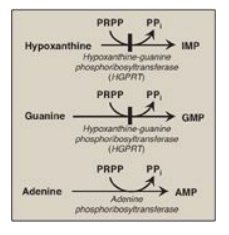
Figure 22.10 Salvage pathways
of purine nucleotide synthesis. [Note: Virtually complete deficiency of HGPRT
results in Lesch-Nyhan syndrome. Partial deficiencies of HGPRT are known. As
the amount of functional enzyme increases, the severity of the symptoms
decreases.]
IMP = inosine monophosphate;
GMP = guanosine monophosphate;
AMP = adenosine monophosphate;
PRPP = 5-phosphoribosyl-1- pyrophosphate;
PPi = pyrophosphate.
2. Lesch-Nyhan syndrome: Lesch-Nyhan is a rare, X-linked
recessive disorder associated with a virtually complete deficiency of HGPRT.
The deficiency results in an inability to salvage hypoxanthine or guanine, from
which excessive amounts of uric acid, the end product of purine degradation,
are then produced. In addition, the lack of this salvage pathway causes
increased PRPP levels and decreased IMP and GMP levels. As a result,
glutamine:phosphoribosylpyrophosphate amidotransferase (the regulated step in
purine synthesis) has excess substrate and decreased inhibitors available, and
de novo purine synthesis is increased. The combination of decreased purine
reutilization and increased purine synthesis results in increased degradation
of purines and the production of large amounts of uric acid, making Lesch-Nyhan
a heritable cause of hyperuricemia. In patients with Lesch-Nyhan syndrome, the
hyperuricemia frequently results in the formation of uric acid stones in the
kidneys (urolithiasis) and the deposition of urate crystals in the joints
(gouty arthritis) and soft tissues. In addition, the syndrome is characterized
by motor dysfunction, cognitive deficits, and behavioral disturbances that
include self-mutilation (for example, biting of lips and fingers) as shown in
Figure 22.11).
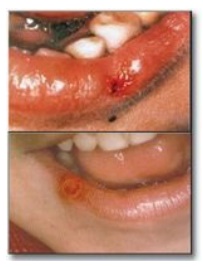
Figure 22.11 Lesions on the lips of Lesch-Nyhan patients caused by self-mutilation.
Related Topics
