Other Nitrogen-Containing Compounds
| Home | | Biochemistry |Chapter: Biochemistry : Conversion of Amino Acids to Specialized Products
Dopamine, norepinephrine, and epinephrine are biologically active (biogenic) amines that are collectively termed catecholamines.
OTHER NITROGEN-CONTAINING COMPOUNDS
A. Catecholamines
Dopamine,
norepinephrine, and epinephrine are biologically active (biogenic) amines that
are collectively termed catecholamines. Dopamine and norepinephrine are
synthesized in the brain and function as neurotransmitters. Norepinephrine is
also synthesized in the adrenal medulla, as is epinephrine.
1. Function: Outside the CNS, norepinephrine and its methylated derivative, epinephrine, are hormone regulators of carbohydrate and lipid metabolism. Norepinephrine and epinephrine are released from storage vesicles in the adrenal medulla in response to fright, exercise, cold, and low levels of blood glucose. They increase the degradation of glycogen and triacylglycerol as well as increase blood pressure and the output of the heart. These effects are part of a coordinated response to prepare the individual for stress, and are often called the “fight-or-flight” reactions.
2. Synthesis: The catecholamines are synthesized from tyrosine, as shown in Figure 21.15. Tyrosine is first hydroxylated by tyrosine hydroxylase to form 3,4-dihydroxyphenylalanine (DOPA) in a reaction analogous to that described for the hydroxylation of phenylalanine. The tetrahydrobiopterin (BH4)-requiring enzyme is abundant in the CNS, the sympathetic ganglia, and the adrenal medulla and is the rate-limiting step of the pathway.
DOPA is decarboxylated in a reaction
requiring PLP to form dopamine, which is hydroxylated by dopamine β-hydroxylase
to yield norepinephrine in a reaction that requires ascorbate (vitamin C) and
copper. Epinephrine is formed from norepinephrine by an N-methylation reaction
using S-adenosylmethionine (SAM) as the methyl donor.
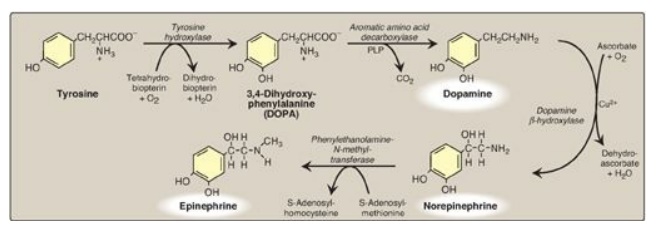
Figure 21.15 Synthesis of
catecholamines. PLP = pyridoxal phosphate; Cu2+ = copper.
Parkinson disease, a
neurodegenerative movement disorder, is due to insufficient dopamine production
as a result of the idiopathic loss of dopamine-producing cells in the brain.
Administration of L-DOPA (levodopa) is the most common treatment. Dopamine
cannot cross the blood brain barrier.
3. Degradation: The
catecholamines are inactivated by oxidative deamination catalyzed by monoamine
oxidase (MAO) and by O-methylation carried out by catechol-O-methyltransferase
(COMT) using SAM as the methyl donor (Figure 21.16). The two reactions can
occur in either order. The aldehyde products of the MAO reaction are oxidized
to the corresponding acids. The metabolic products of these reactions are
excreted in the urine as vanillylmandelic acid (VMA) from epinephrine and
norepinephrine and homovanillic acid from dopamine. [Note: VMA is increased
with pheochromocytomas, rare tumors of the adrenal gland characterized by
excessive production of catecholamines.]
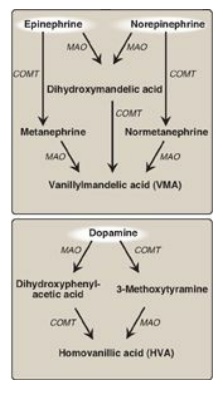
Figure 21.16 Metabolism of the
catecholamines by catechol-O-methyltranferase (COMT) and monoamine oxidase
(MAO).
4. Monoamine oxidase inhibitors: MAO is found in neural and other tissues, such as the intestine and liver. In the neuron, this enzyme oxidatively deaminates and inactivates any excess neurotransmitter molecules (norepinephrine, dopamine, or serotonin) that may leak out of synaptic vesicles when the neuron is at rest.
MAO inhibitors may
irreversibly or reversibly inactivate the enzyme, permitting neurotransmitter
molecules to escape degradation and, therefore, to both accumulate within the
presynaptic neuron and to leak into the synaptic space. This causes activation
of norepinephrine and serotonin receptors and may be responsible for the
antidepressant action of these drugs.
B. Histamine
Histamine is a chemical
messenger that mediates a wide range of cellular responses, including allergic
and inflammatory reactions and gastric acid secretion. A powerful vasodilator,
histamine is formed by decarboxylation of histidine in a reaction requiring PLP
(Figure 21.17). It is secreted by mast cells as a result of allergic reactions
or trauma. Histamine has no clinical applications, but agents that interfere
with the action of histamine have important therapeutic applications.
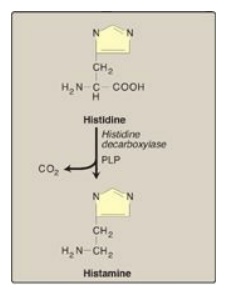
Figure 21.17 Biosynthesis of
histamine. PLP = pyridoxal phosphate.
C. Serotonin
Serotonin, also called
5-hydroxytryptamine (5-HT), is synthesized and stored at several sites in the
body (Figure 21.18). The largest amount by far is found in the intestinal
mucosa. Smaller amounts occur in the CNS, where it functions as a
neurotransmitter, and in platelets. Serotonin is synthesized from tryptophan,
which is hydroxylated in a BH4-requiring reaction analogous to that catalyzed
by phenylalanine hydroxylase. The product, 5-hydroxytryptophan, is
decarboxylated to serotonin, which is degraded by MAO. Serotonin has multiple
physiologic roles including pain perception, regulation of sleep, appetite,
temperature, blood pressure, cognitive functions, and mood (causes a feeling of
well-being). [Note: Selective serotonin reuptake inhibitors (SSRIs) maintain
serotonin levels, thereby functioning as antidepressants.]
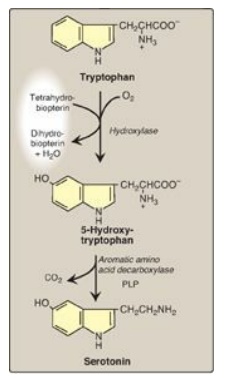
Figure 21.18 Synthesis of serotonin. [Note: Serotonin is converted to melatonin in the pineal gland.] PLP = pyridoxal phosphate.
D. Creatine
Creatine phosphate (also called
phosphocreatine), the phosphorylated derivative of creatine found in muscle, is
a high-energy compound that provides a small but rapidly mobilized reserve of
high-energy phosphates that can be reversibly transferred to adenosine
diphosphate (Figure 21.19) to maintain the intracellular level of adenosine
triphosphate (ATP) during the first few minutes of intense muscular
contraction. [Note: The amount of creatine phosphate in the body is
proportional to the muscle mass.]
1. Synthesis: Creatine is synthesized in liver and kidney tissue from glycine and the guanidino group of arginine, plus a methyl group from SAM (see Figure 21.19). Animal products are dietary sources. Creatine is reversibly phosphorylated to creatine phosphate by creatine kinase, using ATP as the phosphate donor. [Note: The presence of creatine kinase (MB isozyme) in the plasma is indicative of heart damage and is used in the diagnosis of myocardial infarction.]
2. Degradation: Creatine and creatine phosphate
spontaneously cyclize at a slow but constant rate to form creatinine, which is
excreted in the urine. The amount excreted is proportional to the total
creatine phosphate content of the body and, therefore, can be used to estimate
muscle mass. When muscle mass decreases for any reason (for example, from
paralysis or muscular dystrophy), the creatinine content of the urine falls. In
addition, any rise in blood creatinine is a sensitive indicator of kidney
malfunction, because creatinine normally is rapidly removed from the blood and
excreted. A typical adult male excretes about 1–2 g of creatinine per day.
E. Melanin
Melanin is a pigment that occurs in several tissues, particularly the eye, hair, and skin. It is synthesized from tyrosine in melanocytes (pigment-forming cells) of the epidermis. It functions to protect underlying cells from the harmful effects of sunlight. [Note: A defect in melanin production results in oculocutaneous albinism, the most common type being due to defects in copper-containing tyrosinase.]
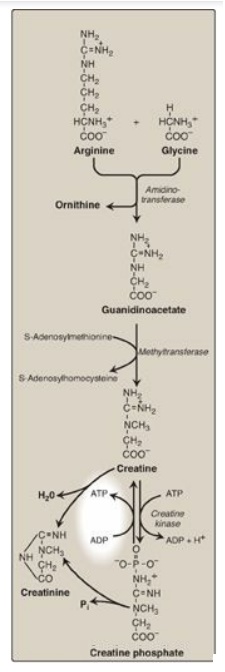
Figure 21.19 Synthesis of
creatine. ADP = adenosine diphosphate; Pi = inorganic phosphate.
Related Topics
