Metabolic Defects in Amino Acid Metabolism
| Home | | Biochemistry |Chapter: Biochemistry : Amino Acid Degradation and Synthesis
Inborn errors of metabolism are commonly caused by mutant genes that generally result in abnormal proteins, most often enzymes.
METABOLIC DEFECTS IN AMINO ACID METABOLISM
Inborn errors of
metabolism are commonly caused by mutant genes that generally result in
abnormal proteins, most often enzymes. The inherited defects may be expressed
as a total loss of enzyme activity or, more frequently, as a partial deficiency
in catalytic activity. Without treatment, the inherited defects of amino acid
metabolism almost invariably result in intellectual disability or other
developmental abnormalities as a consequence of harmful accumulation of
metabolites. Although more than 50 of these disorders have been described, many
are rare, occurring in less than 1 per 250,000 in most populations (Figure
20.13). Collectively, however, they constitute a very significant portion of
pediatric genetic diseases (Figure 20.14). Phenylketonuria (PKU) is an
important disease of amino acid metabolism because it is relatively common and
responds to dietary treatment.
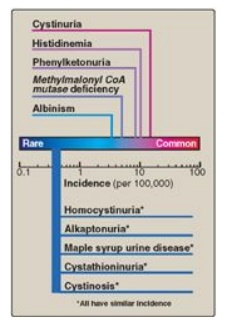
Figure 20.13 Incidence of inherited diseases of amino acid metabolism. [Note: Cystinuria is the most common genetic error of amino acid transport.]
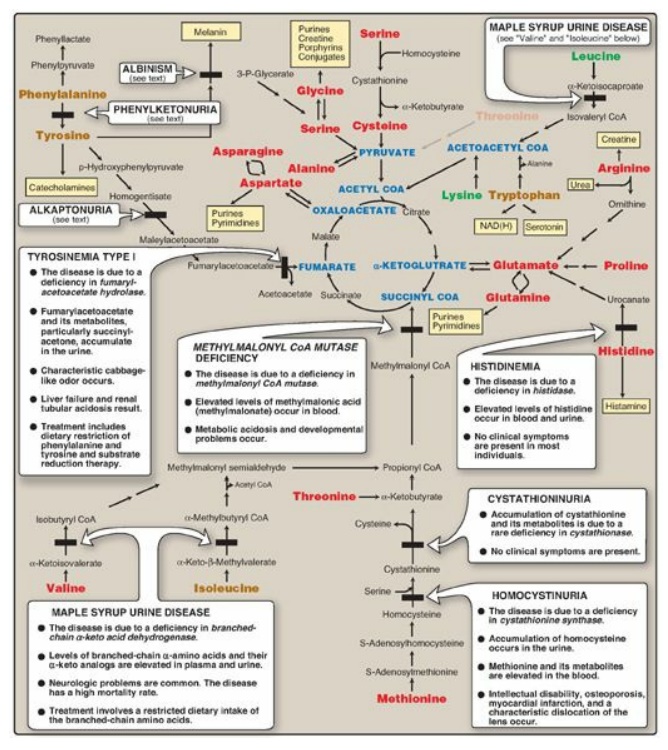
Figure 20.14 Summary of the metabolism of amino acids in humans. Genetically determined enzyme deficiencies are summarized in white boxes. Nitrogen-containing compounds derived from amino acids are shown in small, yellow boxes. Classification of amino acids is color coded: Red = glucogenic; brown = glucogenic and ketogenic; green ketogenic. Compounds in BLUE ALL CAPS are the seven metabolites to which all amino acid metabolism converges. CoA = coenzyme A; NAD(H) = nicotinamide adenine dinucleotide.
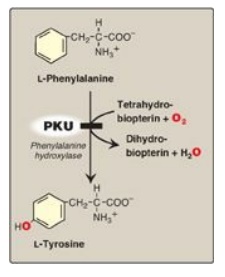
Figure 20.15 A deficiency in
phenylalanine hydroxylase results in the disease phenylketonuria (PKU).
Screening of newborns for a number of treatable
disorders, including those of amino acid metabolism, is done by tandem mass
spectrometry of blood obtained from a heel prick. By law, all states must
screen for over 20 disorders, with some screening for over 30. All states
screen for PKU.
A. Hyperphenylalanemia and phenylketonuria
PKU, caused by a
deficiency of phenylalanine hydroxylase ([PAH] Figure 20.15), is the most
common clinically encountered inborn error of amino acid metabolism (incidence
1:15,000). Biochemically, it is characterized by accumulation of phenylalanine,
resulting in hyperphenylalanemia, and a deficiency of tyrosine. It is treated
by dietary restriction of phenylalanine. Hyperphenylalanemia may also be caused
by deficiencies in any of the several enzymes required to synthesize BH4
or in dihydropteridine reductase, which regenerates BH4 from BH2
(Figure 20.16). Such deficiencies indirectly raise phenylalanine
concentrations, because PAH requires BH4 as a coenzyme. BH4
is also required for tyrosine hydroxylase and tryptophan hydroxylase, which
catalyze reactions leading to the synthesis of neurotransmitters, such as
serotonin and the catecholamines. Simply restricting dietary phenylalanine does
not reverse the central nervous system effects due to deficiencies in
neurotransmitters. Supplementation with BH4 and replacement therapy
with L-3,4-dihydroxyphenylalanine and 5-hydroxytryptophan (products of the
affected tyrosine hydroxylase– and tryptophan hydroxylase–catalyzed reactions)
improves the clinical outcome in these variant forms of hyperphenylalaninemia,
although the response is unpredictable.
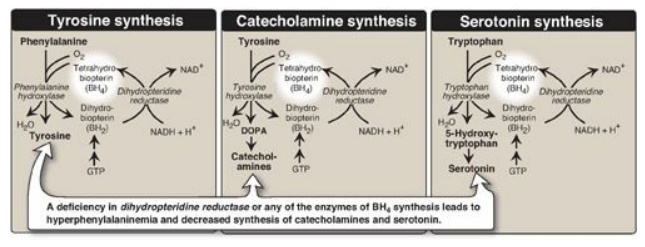
Figure 20.16 Biosynthetic
reactions involving amino acids and tetrahydrobiopterin. [Note: Aromatic amino
acid hydroxylases use BH4 and not PLP (pyridoxal phosphate).] NAD(H)
= nicotinamide adenine dinucleotide; GTP = guanosine triphosphate; DOPA =
3,4-dihydroxyphenylalanine.
1. Characteristics of classic phenylketonuria:
a. Elevated phenylalanine: Phenylalanine is present in high concentrations (ten times normal) in tissues, plasma, and urine. Phenyllactate, phenylacetate, and phenylpyruvate, which are not normally produced in significant amounts in the presence of functional PAH, are also elevated in PKU (Figure 20.17). These metabolites give urine a characteristic musty (“mousey”) odor. [Note: The disease acquired its name from the presence of a phenylpyruvate, a phenylketone in the urine.]
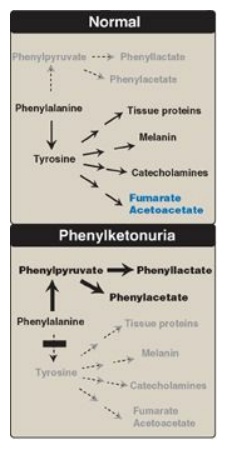
Figure 20.17 Pathways of phenylalanine metabolism in normal individuals and in patients with phenylketonuria.
b. Central nervous system symptoms: Severe intellectual disability,
developmental delay, microcephaly, and seizures are characteristic findings in
untreated PKU. The patient with untreated PKU typically shows symptoms of
intellectual disability by age 1 year and rarely achieves an intelligence
quotient (IQ) greater than 50 (Figure 20.18). [Note: These clinical
manifestations are now rarely seen as a result of neonatal screening programs.]
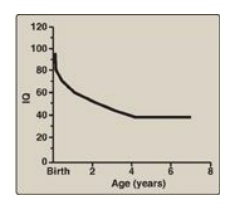
Figure 20.18 Typical intellectual ability in untreated patients of different ages with phenylketonuria. IQ = intelligence quotient.
c. Hypopigmentation: Patients with untreated PKU may show a deficiency of pigmentation (fair hair, light skin color, and blue eyes). The hydroxylation of tyrosine by copper-requiring tyrosinase, which is the first step in the formation of the pigment melanin, is inhibited in PKU.
2. Neonatal screening and diagnosis: Early diagnosis of PKU is important because the disease is treatable by dietary means. Due to the lack of neonatal symptoms, laboratory testing for elevated blood levels of phenylalanine is mandatory for detection. However, the infant with PKU frequently has normal blood levels of phenylalanine at birth because the mother clears increased blood phenylalanine in her affected fetus through the placenta. Normal levels of phenylalanine may persist until the newborn is exposed to 24–48 hours of protein feeding. Thus, screening tests are typically done after this time to avoid false negatives. For newborns with a positive screening test, diagnosis is confirmed through quantitative determination of phenylalanine levels.
3. Prenatal diagnosis: Classic PKU is caused by any of 100
or more different mutations in the gene that codes for PAH. The frequency of
any given mutation varies among populations, and the disease is often doubly
heterozygous (that is, the PAH gene has a different mutation in each allele).
Despite this complexity, prenatal diagnosis is possible.
4. Treatment: Most natural protein contains phenylalanine, an
essential amino acid, and it is impossible to satisfy the body’s protein
requirement without exceeding the phenylalanine limit when ingesting a normal
diet. Therefore, in PKU, blood phenylalanine level is maintained close to the
normal range by feeding synthetic amino acid preparations free of
phenylalanine, supplemented with some natural foods (such as fruits,
vegetables, and certain cereals) selected for their low phenylalanine content.
The amount is adjusted according to the tolerance of the individual as measured
by blood phenylalanine levels. The earlier treatment is started, the more
completely neurologic damage can be prevented. Individuals who are
appropriately treated can have normal intelligence. [Note: Treatment must begin
during the first 7–10 days of life to prevent cognitive impairment.] Because
phenylalanine is an essential amino acid, overzealous treatment that results in
blood phenylalanine levels below normal is avoided. In patients with PKU,
tyrosine cannot be synthesized from phenylalanine, and, therefore, it becomes
an essential amino acid and must be supplied in the diet. Discontinuance of the
phenyalanine-restricted diet in early childhood is associated with poor
performance on IQ tests. Adult PKU patients show deterioration of IQ scores
after discontinuation of the diet (Figure 20.19). Lifelong restriction of
dietary phenylalanine is, therefore, recommended. [Note: Individuals with PKU
are advised to avoid aspartame, an artificial sweetener that contains
phenylalanine.]
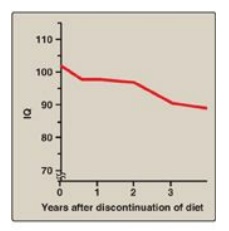
Figure 20.19 Changes in IQ
scores after discontinuation of low-phenylalanine diet in patients with
phenylketonuria. IQ = intelligence quotient.
5. Maternal phenylketonuria: If women with PKU who are not on a
low-phenylalanine diet become pregnant, the offspring are affected with
“maternal PKU syndrome.” High blood phenylalanine levels in the mother cause
microcephaly and congenital heart abnormalities in the fetus (phenylalanine is
a teratogen). Because these developmental responses to high phenylalanine occur
during the first months of pregnancy, dietary control of blood phenylalanine
must begin prior to conception and must be maintained throughout the pregnancy.
B. Maple syrup urine disease
Maple syrup urine
disease (MSUD) is a rare (1:185,000), autosomal recessive disorder in which
there is a partial or complete deficiency in BCKD, a mitochondrial enzyme
complex that oxidatively decarboxylates leucine, isoleucine, and valine (see
Figure 20.10). These BCAAs and their corresponding α-keto acids accumulate in
the blood, causing a toxic effect that interferes with brain functions. The
disease is characterized by feeding problems, vomiting, ketoacidosis, changes
in muscle tone, neurologic problems that can result in coma (primarily due to
the rise in leucine), and a characteristic maple syrup-like odor of the urine
due to the rise in isoleucine. If untreated, the disease is fatal. If treatment
is delayed, intellectual disability results.
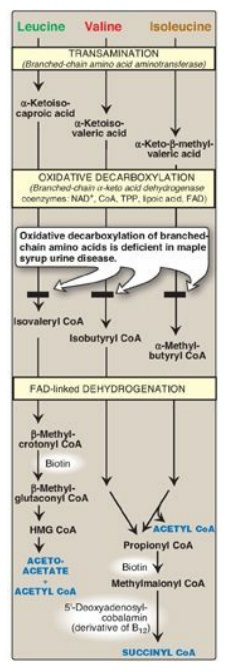
Figure 20.10 Degradation of leucine, valine, and isoleucine. [Note: β-Methylcrotonyl CoA carboxylase is one of four biotinrequiring carboxylases we have encountered. The other three are pyruvate carboxylase, acetyl CoA carboxylase, and propionyl CoA carboxylase.] TPP = thiamine pyrophosphate; FAD = flavin adenine dinucleotide; CoA = coenzyme A; NAD = nicotinamide adenine dinucleotide; HMG = hydroxymethylglutarate.
1. Classification: The term “maple syrup urine
disease” includes a classic type and several variant forms of the disorder. The
classic, neonatal-onset form is the most common type of MSUD. Leukocytes or
cultured skin fibroblasts from these patients show little or no BCKD activity.
Infants with classic MSUD show symptoms within the first several days of life.
If not diagnosed and treated, classic MSUD is lethal in the first weeks of
life. Patients with intermediate forms have a higher level of enzyme activity
(up to 30% of normal). The symptoms are milder and show an onset from infancy
to adolescence. Patients with the rare thiamine-dependent variant of MSUD
respond to large doses of this vitamin.
2. Screening and diagnosis: As with PKU, prenatal diagnosis
and neonatal screening are available, and most affected individuals are
compound heterozygotes.
3. Treatment: MSUD is treated with a synthetic formula that is free of BCAAs, supplemented with limited amounts of leucine, isoleucine, and valine to allow for normal growth and development without producing toxic levels. [Note: Elevated leucine is the cause of the neurologic damage in MSUD, and its level is carefully monitored.] Early diagnosis and lifelong dietary treatment is essential if the child with MSUD is to develop normally. [Note: BCAAs are an important energy source in times of metabolic need, and individuals with MSUD are at risk of decompensation during periods of increased protein catabolism.]
C. Albinism
Albinism refers to a
group of conditions in which a defect in tyrosine metabolism results in a
deficiency in the production of melanin. These defects result in the partial or
full absence of pigment from the skin, hair, and eyes. Albinism appears in
different forms, and it may be inherited by one of several modes: autosomal
recessive (primary mode), autosomal dominant, or X linked. Total absence of
pigment from the hair, eyes, and skin (Figure 20.20) , tyrosinase-negative
oculocutaneous albinism (type 1 albinism), results from an absent or defective
copper-requiring tyrosinase. It is the most severe form of the condition. In
addition to hypopigmentation, affected individuals have vision defects and
photophobia (sunlight hurts their eyes). They are at increased risk for skin
cancer.
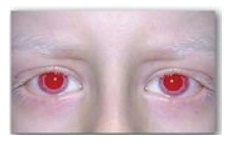
Figure 20.20 Patient with oculocutaneous albinism, showing white eyebrows and lashes and eyes that appear red in color.
D. Homocystinuria
The homocystinurias are
a group of disorders involving defects in the metabolism of Hcy. These
autosomal-recessive diseases are characterized by high plasma and urinary
levels of Hcy and methionine and low levels of cysteine. The most common cause
of homocystinuria is a defect in the enzyme cystathionine β-synthase, which
converts Hcy to cystathionine (Figure 20.21). Individuals who are homozygous
for cystathionine β-synthase deficiency exhibit dislocation of the lens
(ectopia lentis), skeletal anomalies (long limbs and fingers), intellectual
disability, and an increased risk for developing thrombi (blood clots).
Thrombosis is the major cause of early death in these individuals. Patients can
be responsive or nonresponsive to oral administration of pyridoxine (vitamin B6),
which is converted to pyridoxal phosphate, the coenzyme of cystathionine
β-synthase. Vitamin B6–responsive patients usually have a milder and later
onset of clinical symptoms compared with B6-nonresponsive patients. Treatment
includes restriction of methionine intake and supplementation with vitamins B6,
B12, and folate.
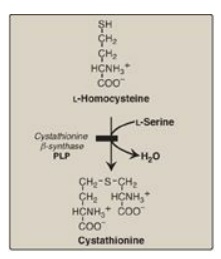
Figure 20.21 Enzyme deficiency
in homocystinuria. PLP = pyridoxal phosphate.
E. Alkaptonuria (alcaptonuria)
Alkaptonuria is a rare
metabolic condition involving a deficiency in homogentisic acid oxidase,
resulting in the accumulation of homogentisic acid (HA), an intermediate in the
degradative pathway of tyrosine. The condition has three characteristic
symptoms: homogentisic aciduria (the urine contains elevated levels of HA,
which is oxidized to a dark pigment on standing, as shown in Figure 20.22A),
large joint arthritis, and deposition of black pigment (ochronosis) in
cartilage and collagenous tissue (Figure 20.22B). Patients with alkaptonuria
are usually asymptomatic until about age 40 years. Dark staining of diapers can
indicate the disease in infants, but usually no symptoms are present until
later in life. Diets low in phenylalanine and tyrosine reduce the levels of HA
and decrease the amount of pigment deposited in body tissues. Although
alkaptonuria is not life-threatening, the associated arthritis may be severely
crippling.
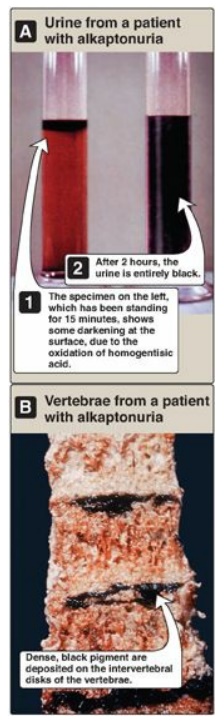
Figure 20.22 A patient with
alkaptonuria. A. Urine. B. Vertebrae.
Related Topics
