Chapter Summary, Questions Answers - Conversion of Amino Acids to Specialized Products
| Home | | Biochemistry |Chapter: Biochemistry : Conversion of Amino Acids to Specialized Products
Amino acids are precursors of many nitrogen-containing compounds including porphyrins, which, in combination with ferrous (Fe2+) iron, form heme.
CHAPTER SUMMARY
Amino acids are
precursors of many nitrogen-containing compounds including porphyrins, which,
in combination with ferrous (Fe2+) iron, form heme (Figure 21.20). The major
sites of heme biosynthesis are the liver, which synthesizes a number of heme
proteins (particularly cytochrome P450 enzymes), and the erythrocyte-producing
cells of the bone marrow, which are active in hemoglobin synthesis. In the
liver, the rate of heme synthesis is highly variable, responding to alterations
in the cellular heme pool caused by fluctuating demands for hemeproteins. In
contrast, heme synthesis in erythroid cells is relatively constant and is
matched to the rate of globin synthesis. Porphyrin synthesis start with glycine
and succinyl coenzyme A. The committed step in heme synthesis is the formation
of δ-aminolevulinic acid (ALA). This reaction is catalyzed by ALA synthase-1
(ALAS1) in liver (inhibited by hemin, the oxidized form of heme that
accumulates in the cell when heme is being underutilized) and ALAS2 in
erythroid tissues (regulated by iron) . Porphyrias are caused by inherited
(primarily autosomal-dominant) or acquired defects in heme synthesis, resulting
in the accumulation and increased excretion of porphyrins or porphyrin
precursors. Enzymic defects early in the pathway cause abdominal pain and
neuropsychiatric symptoms, whereas later defects cause photosensitivity.
Degradation of hemeproteins occurs in the reticuloendothelial system, particularly
in the liver and spleen. The first step in the degradation of heme is the
production by heme oxygenase of the green pigment biliverdin, which is
subsequently reduced to bilirubin. Bilirubin is transported to the liver, where
its solubility is increased by the addition of two molecules of glucuronic
acid. Bilirubin diglucuronide is transported into the bile canaliculi, where it
is first hydrolyzed and reduced by bacteria in the gut to yield urobilinogen,
then oxidized by intestinal bacteria to stercobilin. Jaundice refers to the
yellow color of the skin and sclerae that is caused by deposition of bilirubin,
secondary to increased bilirubin levels in the blood. Three commonly
encountered type of jaundice are hemolytic jaundice, obstructive jaundice, and
hepatocellular jaundice. Other important N-containing compounds derived from
amino acids include the catecholamines (dopamine, norepinephrine, and
epinephrine), creatine, histamine, serotonin, and melanin.
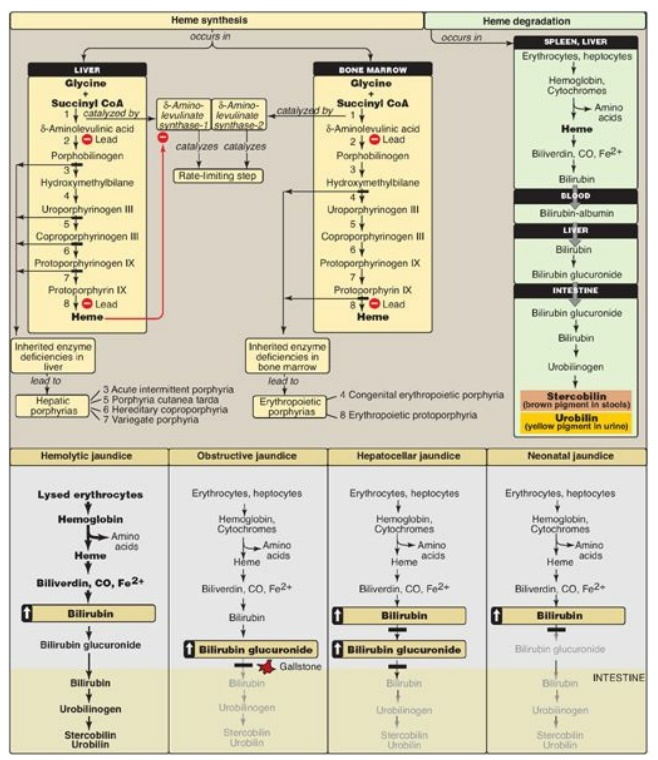
Figure 21.20 Key concept map
for heme metabolism. = Block in the pathway. [Note: Hepatocellular jaundice can
be caused by decreased conjugation of bilirubin or decreased secretion of
conjugated bilirubin into bile.] CoA = coenzyme A; CO = carbon monoxide.
Study Questions
Choose the ONE best answer.
21.1 δ-Aminolevulinic acid synthase activity:
A. catalyzes the committed step in porphyrin
biosynthesis.
B. is decreased by iron
in erythrocytes.
C. is decreased in
liver in individuals treated with certain drugs such as the barbiturate
phenobarbital.
D. occurs in the
cytosol.
E. requires biotin as a
coenzyme.
Correct answer = A. δ-Aminolevulinic acid synthase is
cytosolic and catalyzes the rate-limiting and regulated step of porphyrin
synthesis. It requires pyridoxal phosphate as a coenzyme. Iron increases
production of the erythroid isozyme. The hepatic isozyme is increased in
patients treated with certain drugs.
21.2 A 50-year-old man presented with painful
blisters on the backs of his hands. He was a golf instructor and indicated that
the blisters had erupted shortly after the golfing season began. He did not
have recent exposure to common skin irritants. He had partial complex seizure
disorder that had begun about 3 years earlier after a head injury. The patient
had been taking phenytoin (his only medication) since the onset of the seizure
disorder. He admitted to an average weekly ethanol intake of about 18 12-oz
cans of beer. The patient’s urine was reddish orange. Cultures obtained from
skin lesions failed to grow organisms. A 24-hour urine collection showed
elevated uroporphyrin (1,000 mg; normal, <27mg). The most likely diagnosis
is:
A. acute intermittent
porphyria.
B. congenital
erythropoietic porphyria.
C. erythropoietic
protoporphyria.
D. hereditary
coproporphyria.
E. porphyria cutanea
tarda.
Correct answer = E. The disease is associated with a deficiency in uroporphyrinogen decarboxylase, but clinical expression of the enzyme deficiency is influenced by hepatic injury caused by environmental (for example, ethanol) and infectious (for example, hepatitis B virus) agents. Exposure to sunlight can also be a precipitating factor. Clinical onset is typically during the fourth or fifth decade of life. Porphyrin accumulation leads to cutaneous symptoms and urine that is red to brown.
Treatment of the patient’s
seizure disorder with phenytoin caused increased synthesis of δ-aminolevulinic
acid synthase and, therefore, of uroporphyrinogen, the substrate of the
deficient enzyme. The laboratory and clinical findings are inconsistent with
other porphyrias.
21.3 A patient presents with jaundice, abdominal pain, and nausea. Clinical laboratory studies give the following results:

What is the most likely
cause of the jaundice?
A. Decreased hepatic
conjugation of bilirubin
B. Decreased hepatic
uptake of bilirubin
C. Decreased secretion of bile into the intestine
D. Increased hemolysis
Correct answer = C. The data are consistent with an
obstructive jaundice in which a block in the common bile duct decreases the
secretion of bile containing conjugated bilirubin (CB) into the intestine
(stool will be pale in color). The liver “regurgitates” the CB into the blood
(hyperbilirubinemia). The CB is excreted in the urine (which darkens) and is
referred to as “urinary bilirubin.” Urinary urobilinogen is not present because
its source is intestinal urobilinogen, which is low. The other choices do not
match the data.
21.4 A 2-year-old child
was brought to his pediatrician for evaluation of gastrointestinal problems.
The parents report that the boy has been listless for the last few weeks. Lab
tests reveal a microcytic, hypochromic anemia. Blood lead levels are elevated.
Which of the enzymes listed below is most likely to have higher-than-normal
activity in the liver of this child?
A. δ-Aminolevulinic acid synthase
B. Bilirubiun UDP-glucuronosyltransferase
C. Ferrochelatase
D. Heme oxygenase
E. Porphobilinogen synthase
Correct answer = A. This child has the acquired porphyria of lead poisoning. Lead inhibits δ-aminolevulinic acid dehydratase and, consequently, heme synthesis.
The decrease in heme derepresses δ-aminolevulinic acid synthase-1 (the hepatic
isozyme), resulting in an increase in its activity. The decrease in heme also
results in decreased hemoglobin synthesis, and anemia is seen. Ferrochelatase
is directly inhibited by lead. The other choices are enzymes of heme
degradation.
Related Topics
